Abstract
Activins have been implicated as important regulating factors for many reproductive processes. The aim of this study was to determine the effect of activin A on the development of ovine preantral follicles in vitro. Mechanically isolated preantral follicles (161 ± 2 microm) were cultured for 6 days in the presence of human recombinant activin A (0, 10 and 100 ng/ml). Half of the medium was replaced every second day and follicle diameters were measured. Conditioned medium was subsequently analysed for oestradiol content using a delayed enhancement lanthanide fluorometric immunoassay (DELFIA). At the end of the culture period, follicles were fixed and processed for histology, after which oocyte diameter and granulosa cell death were measured. There was significant follicle growth over 6 days in all groups (p < 0.001). Activin, at both concentrations, increased follicle growth over control levels by Day 6 (p < 0.05). Oocyte diameters were also significantly increased by Day 6 of culture in all groups (p < 0.05), with 100 ng/ml activin increasing oocyte diameter over control levels (p < 0.05). Activin, at both concentrations, increased oestradiol production on Day 2 of culture, but this increase was not sustained during the culture period. Moreover, activin did not have any effect on antrum formation or follicle survival. In conclusion, activin promoted ovine preantral follicle and oocyte growth in vitro, but did not accelerate follicle differentiation over a six-day culture period. These results support a paracrine role for activin A during early oocyte and follicular development.
Similar content being viewed by others
Introduction
Over the last two decades, culture systems have been developed with the aim of growing oocytes from the earliest follicular stages through to maturation and fertilisation. The viability of rodent systems has been demonstrated by the production of live offspring from in vitro grown oocyte-granulosa cell complexes from murine preantral follicles [1] and from the culture of whole preantral follicles [2]. However, progress has been slow in developing these techniques for species with a long follicular growth period, such as humans and domestic species. There are many technical reasons for the lack of progress in these species, but the major problem is that we have very little knowledge of how the oocyte acquires developmental competence during its growth within the follicle. At present the major benefits of culture systems that support the growth and development of immature oocytes are in advancing our knowledge of the co-ordination of oocyte development and granulosa cell differentiation, and understanding the developmental regulation of autocrine/paracrine factors controlling these processes.
The TGF-β superfamily is made up of a number of proteins with the potential to act as intraovarian regulators of ovarian function [3]. These are growth and differentiation factors such as growth differentiation factor-9 (GDF-9), mullerian inhibitory substance (MIS), activins and inhibins [4–6]. Activins are disulphide-linked dimeric glycoproteins, and the subunits inhibin α, inhibin/activin βA and inhibin/activin βB, together with the activin receptors type I, IIA and IIB are expressed in ovarian cells during follicular development [7, 8]. Activin A is composed of two βA subunits, and a role for this factor during early follicular development in sheep is supported by the presence of these peptides in oocytes and granulosa cells from the primordial stage onwards [9]. Activins are thought to play an autocrine/paracrine role in controlling early follicular development by promoting follicular growth and differentiation [5, 10].
The role of activin during early follicular development has been reported in rodent species, where activin has been shown to both promote [11, 12] and inhibit [13] preantral follicle growth and development. These ambiguous actions may be due to developmental regulation. In domestic ruminant species, the role(s) of activin during early follicle development has not been established. Therefore the aims of this study were to determine the effects of activin A on oocyte and granulosa cell development within ovine preantral follicles using a serum free culture system.
Materials and Methods
Preantral Follicle Isolation
Ovine ovaries from random stages of the oestrous cycle were obtained from an abattoir and transported at 25–30°C in M199 (HEPES buffered) media (GIBCO BRL, Life Technologies Ltd. Paisley, Renfrewshire, UK) supplemented with sodium pyruvate (2 mM), glutamine (2 mM), BSA (3 mg/ml), penicillin G (75 μg/ml) and streptomycin (50 μg/ml) (all chemicals from Sigma Chemicals, Poole, Dorset, UK unless otherwise stated). In a laminar flow hood, ovaries without large follicles or corpora lutea on the surface were rinsed with 70% ethanol, and fine slices of ovarian cortex were taken using a scalpel and placed in dissection medium [Leibovitz's medium (GIBCO BRL, Life Technologies Ltd.) supplemented with sodium pyruvate (2 mM), glutamine (2 mM), BSA (Fraction V, 3 mg/ml), penicillin G (75 μg/ml) and streptomycin (50 μg/ml)]. Preantral follicles (161 ± 2 μm) were isolated from the cortical slices under a dissecting microscope using 25G needles. Follicles with an intact basement membrane and even distribution of granulosa and theca layers were selected for culture. The entire process from collection of material from the abattoir to the start of culture was always less than six hours.
Preantral Follicle Culture
For the control group, preantral follicles (n = 34) were cultured individually in 96 well plates (Bibby Sterilin Ltd. Stone, Staffs, UK) in 250 μl of culture medium (McCoy's 5a medium with bicarbonate supplemented with HEPES (20 mM), BSA (0.1 %), L-glutamine (3 mM), penicillin (100 IU/ml), streptomycin (0.1 mg/ml), transferrin (2.5 μg/ml), selenium (4 ng/ml), insulin (10 ng/ml) and L-ascorbic acid, sodium salt (50 μg/ml), all obtained from Sigma Chemicals. Treatments added to the control medium were recombinant human activin A (R&D Systems) at a concentration of 10 ng/ml (n = 33), or 100 ng/ml (n = 32). Isolated preantral follicles were assigned randomly to the treatment groups. Plates were incubated for 6 days in a sterile humidified air atmosphere with 5 % CO2 at 37°C. Each set of cultures (n = 5) took place under identical conditions. Follicle diameters were measured using a crossed micrometer (basement membrane to basement membrane) under the dissection microscope on days 0, 2, 4 and 6. Half of the medium was replaced every second day, and this conditioned medium was stored at -70°C for subsequent analysis of oestradiol content. Any follicles that had lost basement membrane integrity during the first 24 hours of culture were likely to have been damaged during isolation, and were excluded from any further analysis.
Detection of Oestradiol in Culture Medium using a Delayed Enhancement Lanthanide Fluorometric Immunoassay (DELFIA)
Medium from preantral follicles cultured in control medium (n = 14), or in the presence of activin A (10 ng/ml, n = 15; 100 ng/ml, n = 15) was analysed for oestradiol content. Oestradiol was biotinylated by standard procedures using 17β-oestradiol-3-(O-carboxy) methylether and EZ-Link biotin hydrazide (Pierce Warriner UK Ltd, 44 Upper Northgate Street Chester CH1 4EF). Nunc-Immuno Maxisorp 96 well plates were coated with donkey anti-sheep serum by incubating overnight at 4°C in the presence of donkey anti-sheep serum (250 μg/ml) made up in carbonate buffer pH9.6 (100 μl/well). The primary antibody was raised in sheep against 17β-estradiol 6-(O-carboxymethy)-oxime: BSA [14].
Biotinylated oestradiol, follicle conditioned medium, oestradiol standards, and a 1:50,000 dilution of primary antibody, made up in 200 μl of assay buffer, was added to the pre-coated wells of the microtitre plate. The assay buffer consisted of Tris buffer (50 mmol/l; pH 7.8) supplemented with sodium chloride (150 mmol/l), bovine gamma globulin (1%, w/v), Tween-20 (0.01%; v/v), thimerosal (0.0008%; w/v) and diethylenetriamine-penta-acetic acid (0.1 moles/l). After incubating the plates overnight at 4°C they were washed (4×) in a wash buffer consisting of Tris buffer (50 mmol/l; pH 7.8) supplemented with sodium chloride (150 mmol/l), Tween-20 (0.01%; v/v) and thimerosal (0.0008%; w/v) before adding 100 μl of assay buffer containing 100 ng/ml europium labelled streptavidin (Perkin-Elmer Life Sciences address) followed by incubation at room temperature for 1 h with shaking. The plates were washed 4× in wash buffer before addition of 200 μl of DELFIA enhancement solution (Perkin-Elmer Life Sciences) to each well of the microtitre plate, and incubated for a further 5 mins with shaking at room temperature. The plates were analysed on a Victor 2 Multilabel Counter (Perkin-Elmer Life Sciences) by time resolved fluorimetry. The emission and excitation wavelengths were 615 nm and 340 nm respectively with a time delay of 400 μs. The inter- and intra-assay coefficients of variation were 13.2 and 9.6 % respectively. The minimum detectable level was 8.5 pg oestradiol per well.
Histological Assessment
At the end of the culture period, follicles were fixed overnight in Bouin's solution and dehydrated in ethanol (70%, 80%, 90%, 100%). Follicles were visualised during processing by addition of eosin to the 70% ethanol. Absolute ethanol was replaced with cedar wood oil for a minimum of 24 hours, then the oil was cleared from the follicles using toluene for 30 min. Follicles were embedded in paraffin wax (60°C), with changes every hour for 4 h to remove all traces of toluene. The samples were sectioned (6 μm) and mounted on gelatin-coated slides, and allowed to dry overnight at 37°C before staining with Haemotoxylin and Eosin.
Histological measurements and observations were made under the light microscope with a crossed micrometer (Graticules Ltd.). The section containing the oocyte nucleolus, or if this was absent, the largest cross-section of the oocyte was used for observations and measurements. Oocyte diameters were measured, and granulosa cell death within a follicle was assessed by counting the number of pyknotic nuclei and expressing them as a percentage of the total number of granulosa cells. Only follicles that had maintained basement membrane integrity during culture were used for further analysis.
Statistical Analyses
Mean follicle diameter and oestradiol production on every second culture day were compared between experimental groups using a one-way ANOVA, with subsequent 2-sample t-tests to allow for individual comparisons between groups. The percentage of pyknotic granulosa cell nuclei were also compared between treatment groups using ANOVA and t-tests. A general linear model was applied to all data to take into account any differences between individual cultures. Oocyte diameters from freshly isolated and cultured preantral follicles were compared using a one-way ANOVA, with subsequent 2-sample t-tests.
Results
Follicle Growth
Follicles were cultured for 6 days in control medium (n = 34) or in medium containing activin A (10 ng/ml, n = 33; 100 ng/ml, n = 32). As illustrated in Figure 1a, there was a significant increase in follicular diameter during culture in all treatment groups (p < 0.001). At the end of the culture period, there was a significant difference between groups in terms of follicular diameter (p < 0.05). Activin, at both concentrations, stimulated follicular growth over control levels (p < 0.05).
(A) Growth of ovine preantral follicles in control medium (open circles) or in the presence of activin A (10 ng/ml, closed circles; 100 ng/ml, triangles). Values are mean ± SEM. Growth is significant between Days 0 and 6 within treatment groups (p < 0.001). Activin A, at both concentrations, stimulated follicular growth over control levels by Day 6 of culture (p < 0.05). (B) Oestradiol production by ovine preantral follicles cultured in control medium (black bars), or in the presence of activin A [10 ng/ml (open bars), 100 ng/ml (striped bars)]. Values are mean ± SEM. Both concentrations of activin increased oestradiol concentration over control levels on Day 2 (p < 0.01), but there were no differences between groups on Day 4 and 6 of culture.
Oestradiol Production
Conditioned medium from control follicles (n = 14) and activin-treated follicles (10 ng/ml, n = 15; 100 ng/ml, n = 15) was analysed for oestradiol concentration on days 2, 4 and 6 of culture. Activin, at both 10 ng/ml and 100 ng/ml, increased oestradiol production on Day 2 of culture, compared with controls (p < 0.01). However, by day 4 and 6 of culture, there was no significant difference in oestradiol production between activin-treated follicles and controls (Figure 1b).
Oocyte Growth
Histological observations were made using freshly isolated follicles (Day 0), and on Day 6 of culture using control follicles (n = 25) and follicles treated with activin A (10 ng/ml, n = 25; 100 ng/ml, n = 25). Oocyte diameter increased from Day 0 to Day 6 in all culture groups (p < 0.05) (Figure 2). Moreover, activin, at a concentration of 100 ng/ml, increased oocyte diameter over control levels on Day 6 of culture (p < 0.05) (Figure 2).
Oocyte diameters measured on Day 0, and in Day 6 cultured control follicles and follicles treated with activin A. Values are mean ± SEM. Oocyte diameter increased from Day 0 to Day 6 in all culture groups (p < 0.05). Activin A, at a concentration of 100 ng/ml, increased oocyte diameter over control levels on Day 6 of culture (p < 0.05).
Antrum Formation and Granulosa Cell Death
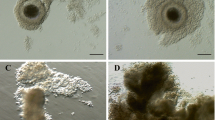
From histological observations, no significant differences in the incidence of antrum formation or the percentage of pyknotic granulosa cell nuclei were found between activin-treated follicles and controls on Day 6 of culture (Table 1). Preantral follicles fixed on Day 6 of culture, showing antrum formation (A) and pyknotic nuclei (B), are represented in Figure 3.
Discussion
Previous in vitro studies have failed to produce competent oocytes from preantral follicles in species such as humans and domestic ruminants. Improved knowledge of the role of paracrine factors in regulating early oocyte and follicle development is important for optimisation of culture systems for the production of developmentally competent oocytes. In the current study, a serum-free culture system was used to investigate the effect of activin on early follicular growth, differentiation and survival in vitro.
In rodents, the precise role of activin during early follicular development is unclear. For example, activin increases the size of preantral follicles and promotes antrum formation in immature mice [12, 15]. By contrast, activin does not stimulate growth of preantral follicles in adult mice, and blocks the effect of FSH on follicular growth [15]. Moreover, it has been reported that activin produced by secondary mouse follicles suppresses the growth of primary follicles in vitro [13], and it was hypothesised that a local decline in activin as a result of atresia of secondary follicles may be responsible for promotion of early folliculogenesis in response to circulating FSH [13]. Thus, according to the rodent culture models, there appears to be age-and stage-dependant effects of activin on follicular development. In the present study, medium-sized and large preantral follicles (< 200 μm diameter) were isolated from ovaries obtained from animals of unknown age; therefore investigation into any similar age- or stage-dependent effects of activin in sheep follicles was out-with the scope of this investigation.
TGF-β superfamily members are likely to play a significant role in folliculogenesis in ruminants, as mutations in the TBF-β signalling system have been implicated in the increased ovulation rate observed in Booroola and Inverdale sheep [16, 17]. Although activin A has been shown to promote oestradiol synthesis whilst suppressing progesterone in ovine granulosa cell culture [18], there is little information on the role of this factor during the early stages of ovine follicle development.
As a member of the TGF-β family, activin executes its actions through a group of intracellular signal transducers, the Smad protein family [19]. There is evidence of an activin signal transduction pathway in oocytes, as activin receptors and Smads 2, 4 and 8 are present [20]. Granulosa cells also express activin receptors, as well as Smads 2 and 4 [20]. Thus activin is capable of directly influencing early oocyte and follicle development, as supported by the present study. The presence of receptors on both the oocyte and somatic cells may provide an explanation for the dose-dependent effect of activin on oocyte growth presented here. However, further investigations will be necessary to ascertain activin receptor levels during ovine follicle development in vitro.
Ovine preantral follicles have previously been developed to the antral stage in culture medium containing serum and high levels of FSH [21]. In serum-free conditions, ovine oocyte-granulosa complexes from fresh and frozen-thawed tissue have been grown to the antral stage during long term culture, and these have produced increasing levels of oestradiol [22]. However, these studies have proved to be unsatisfactory in terms of producing oocytes capable of maturation and further development. Due to the protracted nature of preantral follicle development in domestic ruminants, the ideal culture system should accelerate oocyte development, without inducing inappropriate follicular differentiation. Androgens have been shown to increase murine preantral follicle growth and oestradiol secretion [23, 24]. However, we have recently shown that androstenedione increases follicular differentiation but decreases oocyte survival within ovine preantral follicles [25]. A recent study using rat preantral follicles found that activin increased FSH-induced follicular inhibin-α expression (a marker of differentiation) during a 72 hour culture period [6]. In contrast, another TGF-β family member, MIS, was found to promote follicle growth and proliferation, without potentiating FSH-induced follicular differentiation [6]. In the current investigation, although activin promoted oestradiol production by Day 2 of culture, this increase was not sustained during the subsequent 4 days of culture. It should be noted that growth of ovine preantral and early antral follicles is thought to be independent of gonadotrophins, and expression of steroidogenic enzymes such as cytochrome P450 aromatase increase during antral follicle development [26]. This would provide an explanation for the low oestradiol levels produced by preantral follicles in the present study. Further investigations into the effect of activin in combination with FSH, using an extended culture system, will be useful to determine the role of activin in steroidogenesis in ovine follicles after antrum formation. As sheep follicles have a much longer growth period compared with rodent follicles, there are likely to be species differences in the role of paracrine factors, including activin, during preantral follicle growth and differentiation.
In mice, a combination of FSH and insulin, which act synergistically to promote differentiation and function of granulosa cells [27–29], causes precocious differentiation of immature murine oocyte-associated granulosa cells in culture [30]. Interestingly, this differentiation was found to be associated with reduced competence of oocytes to undergo fertilisation and pre-implantation development [30]. In the present study, activin was found to have a positive effect on both follicle and oocyte growth after six days. Moreover, activin did not promote inappropriate differentiation, as oestradiol concentrations were not increased over control levels for the majority of the culture period. In addition, activin did not significantly increase the incidence of antrum formation. Future studies employing an extended culture period will be important to assess the developmental potential of oocytes after treatment with activin.
The culture system reported here has previously been shown to maintain bovine preantral follicle survival and basement membrane integrity over six days [31]. Here, using ovine follicles, we have shown that the incidence of granulosa cell death was low across all culture groups. Thus the increased follicular growth observed in the presence of activin was likely to be due to granulosa cell proliferation, rather than a result of increased atresia of control follicles.
In summary, this study has shown that activin promotes ovine preantral follicle and oocyte development over a six day culture period, without accelerating follicular differentiation. Improved knowledge of the species- and stage-specific roles of paracrine factors in the co-ordination of oocyte development and somatic cell differentiation is essential for the development of culture systems capable of supporting oocyte development and acquisition of developmental competence. A technique for obtaining a source of homogeneous mature oocytes from ovine ovaries would provide a model for in vitro maturation and fertilisation systems for human oocytes, as well as permitting investigations into postovulatory and embryonic development.
References
Eppig JJ, Schroeder AC: Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989, 41: 268-276.
Spears N, Boland NI, Murray AA, Gosden RG: Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994, 9: 527-532.
Armstrong DG, Webb R: Ovarian follicular dominance: the role of intraovarian growth factors and novel proteins. Rev Reprod. 1997, 2: 139-146. 10.1530/revreprod/2.3.139.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM: Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996, 383: 531-535. 10.1038/383531a0.
Findlay JK: An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biol Reprod. 1993, 48: 15-23.
McGee EA, Smith R, Spears N, Nachtigal MW, Ingraham H, Hsueh AJ: Mullerian inhibitory substance induces growth of rat preantral ovarian follicles. Biol Reprod. 2001, 64: 293-298.
Cameron VA, Nishimura E, Mathews LS, Lewis KA, Sawchenko PE, Vale WW: Hybridization histochemical localization of activin receptor subtypes in rat brain, pituitary, ovary, and testis. Endocrinology. 1994, 134: 799-808. 10.1210/en.134.2.799.
Roberts VJ, Barth S, el Roeiy A, Yen SS: Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. J Clin Endocr Metab. 1993, 77: 1402-1410. 10.1210/jc.77.5.1402.
McNatty KP, Heath DA, Lundy T, Fidler AE, Quirke L, O'Connell A, Smith P, Groome N, Tisdall DJ: Control of early ovarian follicular development. J Reprod Fert Suppl. 1999, 54: 3-16.
Mather JP, Moore A, Li RH: Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med. 1997, 215: 209-222.
Li R, Phillips DM, Mather JP: Activin promotes ovarian follicle development in vitro. Endocrinology. 1995, 136: 849-856. 10.1210/en.136.3.849.
Zhao J, Taverne MA, van der Weijden GC, Bevers MM, Van den Hurk R: Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod. 2001, 65: 967-977.
Mizunuma H, Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Yokota H, Ibuki Y, Hasegawa Y: Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology. 1999, 140: 37-42. 10.1210/en.140.1.37.
Webb R, Baxter G, McBride D, Nordblom GD, Shaw MP: The measurement of testosterone and oestradiol-17 beta using iodinated tracers and incorporating an affinity chromatography extraction procedure. J Steroid Biochem. 1985, 23: 1043-1051. 10.1016/0022-4731(85)90065-2.
Yokota H, Yamada K, Liu X, Kobayashi J, Abe Y, Mizunuma H, Ibuki Y: Paradoxical action of activin A on folliculogenesis in immature and adult mice. Endocrinology. 1997, 138: 4572-4576. 10.1210/en.138.11.4572.
Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, et al: Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000, 25: 279-283. 10.1038/77033.
Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan JC, O'Connell AR, et al: Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod. 2001, 64: 1225-1235.
Shidaifat F, Khamas W, Hailat N: Activin-A differentially regulates steroidogenesis by sheep granulosa cells. Res Vet Sci. 2001, 71: 23-25. 10.1053/rvsc.2001.0479.
Heldin CH, Miyazono K, ten Dijke P: TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997, 390: 465-471. 10.1038/37284.
Findlay JK, Drummond AE, Dyson M, Baillie AJ, Robertson DM, Ethier JF: Production and actions of inhibin and activin during folliculogenesis in the rat. Mol Cell Endocrinol. 2001, 180: 139-144. 10.1016/S0303-7207(01)00521-4.
Cecconi S, Barboni B, Coccia M, Mattioli M: In vitro development of sheep preantral follicles. Biol Reprod. 1999, 60: 594-601.
Newton H, Picton H, Gosden RG: In vitro growth of oocyte-granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fert. 1999, 115: 141-150.
Murray AA, Gosden RG, Allison V, Spears N: Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fert. 1998, 113: 27-33.
Spears N, Murray AA, Allison V, Boland NI, Gosden RG: Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fert. 1998, 113: 19-26.
Thomas FH, Campbell BK, Baird DT, Telfer EE: Oocyte development and granulosa cell differentiation in ovine preantral follicles in vitro : Effect of androstenedione and activin A. Reproduction Abstract Series. 2002, 29: Abstract 4-
Webb R, Campbell BK, Garverick HA, Gong JG, Gutierrez CG, Armstrong DG: Molecular mechanisms regulating follicular recruitment and selection. J Reprod Fertil Suppl. 1999, 54: 33-48.
Amsterdam A, May JV, Schomberg DW: Synergistic effect of insulin and follicle-stimulating hormone on biochemical and morphological differentiation of porcine granulosa cells in vitro. Biol Reprod. 1988, 39: 379-390.
Gutierrez CG, Campbell BK, Webb R: Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol Reprod. 1997, 56: 608-616.
May JV, Schomberg DW: Granulosa cell differentiation in vitro : effect of insulin on growth and functional integrity. Biol Reprod. 1997, 25: 421-431.
Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S: Factors affecting the developmental competence of mouse oocytes grown in vitro : follicle-stimulating hormone and insulin. Biol Reprod. 1998, 59: 1445-1453.
Thomas FH, Leask R, Srsen V, Riley SC, Spears N, Telfer EE: Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction. 2001, 122: 487-495. 10.1530/reprod/122.3.487.
Acknowledgements
The authors thank David Thomas for advice on the statistical analysis, and Debbie Bruce and John Binnie for help with histological preparation and follicle dissection. This study was supported by the MRC.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Thomas, F.H., Armstrong, D.G. & Telfer, E.E. Activin promotes oocyte development in ovine preantral follicles in vitro. Reprod Biol Endocrinol 1, 76 (2003). https://doi.org/10.1186/1477-7827-1-76
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-76