Abstract
Background
The potential of secretory leukocyte protease inhibitor (SLPI) as a biomarker for colorectal cancer was studied. A prospective, randomized, controlled, clinical trial was conducted in 2013 and 2014 to confirm whether the expression of SLPI correlates with prognosis and metastasis in colorectal cancer patients.
Methods
Immunohistochemistry was used to detect SLPI expression in colorectal cancer. The expression of SLPI was scored by two pathologists independently. Statistical analysis of the data was performed using a Χ2 test to investigate the influence of SLPI on the pathologic characteristics of colorectal cancer.
Results
Compared with normal tissue, SLPI was overexpressed in colorectal cancer tissue. Overexpression of SLPI correlated with different grades (moderate or good differentiation: 2.7% low expression versus 97.3% high expression, low differentiation: 41.7% low expression versus 58.3% high expression), TNM stage (I or II: 4.2% low expression versus 95.8% high expression; III or IV: 19.7% low expression versus 80.3% high expression), lymphatic metastasis (18.6% low expression versus 81.4% high expression) and distal metastasis (86.5% low expression versus 13.5% high expression), but not with patient age or sex (P = 0.613, P = 0.871).
Conclusions
Upregulated SLPI correlates with aggressive pathologic characteristics of colorectal cancer; SLPI could be used as an indicator of progression and metastasis in patients with colorectal cancer.
Similar content being viewed by others
Background
Colorectal cancer is one of the most common malignancies worldwide and is a major cause of cancer-related deaths [1]. Survival rates of patients with colorectal cancer have increased in the past few years, possibly as a result of earlier diagnosis and improved treatment regimens; nonetheless, approximately 30% to 50% of patients who undergo curative resection subsequently experience local tumor recurrence or metastasis [2, 3].
The secretory leukocyte protease inhibitor (SLPI) is an 11.7-kDa, nonglycosylated, single-chain protein that is produced by different cell types, including lung epithelial cells, secretory cells of the salivary glands, and various host inflammatory and immune cells, such as macrophages, neutrophils, and B lymphocytes [4–7]. However, its potential as biomarker for colorectal cancer has not been studied.
This study was a prospective, randomized, controlled, clinical trial conducted in 2013 and 2014 to confirm whether SLPI expression correlates with prognosis and metastasis in colorectal cancer patients.
Methods
Tumor tissues
A uniform cohort of 296 patients (162 men and 134 women) with colorectal cancer (6 with stage I, 112 with stage II, 132 with stage III and 46 with stage IV cancer) diagnosed between January 2013 and March 2014 were selected. All patients provided their consent for participation in the study, which was approved by the local ethics committee. The age of the patients ranged from 24 to 87 years (mean: 53.3 years). Table 1 shows patients’ detailed information. Two cores of normal mucosa and two cores of tumor tissue for each patient were paraffin-embedded in ordered microarrays. Tumor microarrays were sectioned in preparation for immunohistochemical staining. Staining intensity was scored by two pathologists separately. A score of 1 was used to signify low intensity, a score of 2 signified intermediate intensity, and a score 3 signified high intensity. A score of 0 to 2 indicates low SLPI expression and a score of 3 indicates high SLPI expression [8].
Tissue preparation and staining
Specimens were fixed in 10% paraformaldehyde. Paraffin-embedded tissue microarrays were incubated at 60°C prior to de-waxing and rehydration. Antigen retrieval was achieved by placing sections in 10 μM citric acid (pH 6) and microwaving for 15 minutes. Endogenous peroxidases were quenched in 15 ml hydrogen peroxide and 185 ml of water. Samples were washed again with PBS prior to treatment with Starting Block (Thermo Scientific, Rockford, IL) for 10 minutes. Tissues were incubated in mouse anti-human SLPI monoclonal antibody (1:50; Santa Cruz, American). The primary antibodies were diluted in PBT (10× PBS, 10% BSA, 10% Triton X-100 in double distilled H2O) at 4°C overnight. Samples were washed with PBS on the following day, incubated in secondary antibody (1:1000), washed and then treated using a 3,3'-diaminobenzidine (DAB) peroxidase staining kit (Shanghai Biotechnology Company, NO132, Xuhui District, Shanghai City, China) as per the manufacturer’s protocol. For detection, a DAB peroxidase detection kit was used and color development was monitored using an optical microscope. The development process was terminated by removing DAB and rinsing the sections with ddH2O for 1 min prior to counterstaining with hematoxylin.
Statistical analysis
Data are representative of three independent experiments. SPSS20.0 software was used for statistical analysis. Statistical analysis of the data were performed using the Χ2 test. The differences were considered significant at P < 0.05.
Results
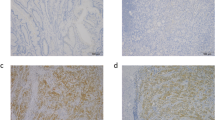
In Figure 1, we can see negative expression of SLPI in normal colon tissue (Figure 1A) and positive expression of SLPI in poorly and well differentiated colon cancer (Figure 1B,C).
As shown in Table 1, the expression of SLPI was not correlated with age or sex (P = 0.613, P = 0.871). The positive expression of SLPI was positively correlated with the degree of tumor differentiation (P < 0.05). There is significant correlation between SLPI and the TNM stage of the tumor. The rate of positive expression in patients with stage III or IV cancer was significantly higher than that in stage I or II (P < 0.05). Moreover, the expression of SLPI in patients with distant metastasis is higher than that without metastases (P < 0.05).
Discussion
The secretory leukocyte protease inhibitor gene is located on chromosome 20q12-13.1 in human beings and on chromosome 2H in mice, with a similar exon-intron configuration [8]. Although physiologically unique and designated as a protease inhibitor, SLPI has the structural characteristics of a WFDC domain. Chromosome 20q13 was recently recognized as the WFDC locus, containing genes encoding 14 WFDC-type protease inhibitors [9]. Secretory leukocyte protease inhibitor, which has antimicrobial and anti-protease functions, belongs to the whey acidic protein four-disulfide core family of proteins, and is also produced in cancer tissues, but its role in cancer is not well understood [10].
Bouchard et al.[11] reported that SLPI expression is highly upregulated in pancreatic, papillary thyroid, uterine cervix, endometrial, and ovarian cancer; by contrast, SLPI is underexpressed in nasopharyngeal carcinoma, bladder tumors, and some breast carcinomas, although overexpression of this protein correlates with more invasive forms of breast carcinoma. Devoogdt et al.[12] reported that several factors, such as inflammatory cytokines and steroid hormones, affect SLPI gene expression. Hoskins et al.[13] reported that SLPI stimulates ovarian cancer invasion, modulated in part by its serine protease inhibitory activity, attenuating MMP-9 release.
This study investigated the potential clinical utility of SLPI to serve as a prognostic and metastasis-predictive biomarker in patients with colorectal cancer. We performed independent validation experiments using a large cohort of samples from 296 patients with colorectal cancer. These data provide strong evidence that SLPI is overexpressed in colorectal cancer tissue. Overexpression of SLPI is correlated with tumor grade and TNM stage, but not with patient age or sex. Our data are of particular interest because they highlight that overexpression of SLPI provides a predictor of lymphatic metastasis and distant metastasis.
Conclusions
The high expression level of SLPI detected by immunohistochemistry in colorectal cancer showed that it correlated with poorly differentiated colorectal cancer with a TNM stage of III or IV, lymphatic metastasis and distal metastasis. Collectively, these results indicate that evaluation of SLPI expression in patients with colorectal cancer presents a clinically promising biomarker that can facilitate disease risk assessment and severity in patients with colorectal cancer. It could be used as an indicator for progression and metastasis of colorectal cancer. However, further studies should be undertaken to verify whether SLPI can become a new indicator for colorectal cancer recurrence and survival of patients.
Abbreviations
- BSA:
-
bovine serum albumin
- DAB:
-
3,3'-diaminobenzidine
- PBS:
-
phosphate buffered saline
- PBT:
-
10× PBS, 10% BSA, 10% Triton X-100 in double distilled H2O
- SLPI:
-
secretory leukocyte protease inhibitor.
References
Siegel R, Naishadham D, Jemal A: Cancer statistics 2012. CA Cancer J Clin. 2012, 62: 10-29. 10.3322/caac.20138.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR: Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012, 143: 844-857. 10.1053/j.gastro.2012.06.001.
Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A: Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014, 259 (4): 735-743. 10.1097/SLA.0b013e3182a6909d.
Abe T, Kobayashi N, Yoshimura K, Trapnell BC, Kim H, Hubbard RC, Brewer MT, Thompson RC, Crystal RG: Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest. 1991, 87: 2207-2215. 10.1172/JCI115255.
Franken C, Meijer CJ, Dijkman JH: Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989, 37: 493-498. 10.1177/37.4.2926127.
Jin FY, Nathan C, Radzioch D, Ding A: Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997, 88: 417-426. 10.1016/S0092-8674(00)81880-2.
Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P: The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res. 2006, 66 (6): 3062-3070. 10.1158/0008-5472.CAN-05-2638.
Kikuchi T, Abe T, Hoshi S, Matsubara N, Tominaga Y, Satoh K, Nukiwa T: Structure of the murine secretory leukoprotease inhibitor (Slpi) gene and chromosomal localization of the human and murine SLPI genes. Am J Respir Cell Mol Biol. 1998, 19: 875-880. 10.1165/ajrcmb.19.6.3314.
Clauss A, Lilja H, Lundwall A: A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein. Biochem J. 2002, 368: 233-242. 10.1042/BJ20020869.
King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK: LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011, 71 (12): 4260-4268. 10.1158/0008-5472.CAN-10-4637.
Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM: Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. 2006, 7: 167-174. 10.1016/S1470-2045(06)70579-4.
Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P: Secretory leukocyte protease inhibitor in cancer development. Ann NY Acad Sci. 2004, 1028: 380-389. 10.1196/annals.1322.044.
Hoskins E, Rodriguez-Canales J, Hewitt SM, Elmasri W, Han J, Han S, Davidson B, Kohn EC: Paracrine SLPI secretion upregulates MMP-9 transcription and secretion in ovarian cancer cells. Gynecol Oncol. 2011, 122 (3): 656-662. 10.1016/j.ygyno.2011.04.052.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GL and JY conceived and designed the experiment. GL wrote the paper. JY analyzed the data. YZ, WZ, and DS contributed reagents, materials, and analysis tools. BX revised the manuscript. LW performed the experiments. All authors read and approved the final manuscript.
Guiying Liu, Jingyan Yang contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, G., Yang, J., Zhao, Y. et al. Expression of secretory leukocyte protease inhibitor detected by immunohistochemistry correlating with prognosis and metastasis in colorectal cancer. World J Surg Onc 12, 369 (2014). https://doi.org/10.1186/1477-7819-12-369
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-12-369