Abstract
Herceptin is widely regarded as the most important development in the treatment of breast cancer since Tamoxifen and the development of the multidisciplinary team (MDT). It is particularly exciting from an oncological polint of view as it represents success in the emerging field of specific targeted therapies to specific molecular abnormalities in tumour cells. This review will focus on the nature of the Her2 overexpression and the role of herceptin in the treatment of early breast cancer.
Similar content being viewed by others
Introduction
The structure of HER-2
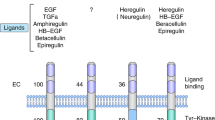
The HER-2 (neu/cerB2) proto-oncogene is located on chromosome 17 and encodes a 185 kDa transmembrane tyrosine kinase receptor which exhibits extensive homology to the epidermal growth factor receptor [1–5]. It consists of an extracellular domain, a transmembrane domain and a cytoplasmic tyrosine kinase domain via which it exerts it's intracellular action (figure 1).
It appears that the receptor is constitutionally active but on interaction with it's ligand [6, 7], heterodimerisation occurs activating the receptor further [8–11].
The role of Her-2 in health and disease
In normal cells, HER-2 plays a key role in cellular growth factor signal transduction and is also involved in the regulation of cell growth, survival and differentiation [12]. The Her-2 oncogene can be activated by point mutations, gene amplification or over-expression and it is now well accepted that this predicts for a poor outcome in mammalian breast cancer [13, 14] and is associated with ER negativity and nodal/brain metastasis. Her-2 overexpression occurs in approximately 20–30% of breast cancers [15] and is also overexpressed in lung, ovarian and gastric adenocarcinomas [16].
HER2 over expression has also been found to be an independent prognostic predictor of overall survival and time to relapse [15, 17, 18]. Several authors have reported a higher frequency of HER2 over expression in ductal carcinoma in situ (DCIS) compared with invasive cancer [19, 20] Similarly, Liu et al [20] have observed HER2 overexpression as determined by gene amplification using polymerase-chain reaction (PCR)-based techniques in 48% of in situ carcinomas compared with 21% of stage II invasive breast tumours. These results were also confirmed using immunohistochemical techniques (IHC). Allred et al [19] reported HER2 over expression as measured by IHC in 56% of cases of pure DCIS (77% in comedo lesions), in 22% of infiltrating ductal carcinomas (IDC) associated with DCIS and 15% of IDC not associated with DCIS. None of the hyperplastic/dysplastic breast lesions overexpressed HER2. These observations suggest that HER2 plays a significant role in the genetic initiation of mammary carcinogenesis rather than in disease progression [21]. HER2 expression also appears to show good concordance between the primary tumour and both synchronous and subsequent metastasis in both the intensity and pattern of IHC staining [22, 23] suggesting that the oncogenic overexpression remains stable.
Clinical Her-2 Testing
Accurate measurement of HER2 amplification and/or overexpression is vital due to the prognostic and potential therapeutic implications of being HER-2 positive.
Two main methods exist for the determination of HER2 gene amplification and protein expression in breast cancer specimens, fluorescence in situ hybridisation or FISH (direct or indirect) and IHC. The former measures gene amplification in breast cancer specimens whilst the latter measures protein expression.
In the FISH technique, fluorescence labelled cDNA probes for HER2 (chromosome 17 q11.2–q12.0) and chromosome 17 centromeres [(chromosome enumeration probe 17 (CEP17)] are used. The HER2 gene appears as a red/orange signal and the CEP17 appears as a green. A ratio of HER2:CEP17 copy number > 2 denotes amplification when taken over an average of at least 60 invasive cancer cells.
In the IHC technique, membrane staining of malignant cells is assessed using the appropriate antibody in fixed tumour blocks. It is a semi-quantitative technique with the intensity of staining reflecting the amount of protein present. From the therapeutic standpoint, the recommended scoring system to evaluate IHC staining is shown in Table 1 below.
This system has been used in pivotal trials [24] evaluating the efficacy of a humanised anti-HER2 monoclonal antibody therapy in woman with advanced breast cancer and has been approved by the appropriate authorities in the USA and the EU.
Ridolfi et al (23) tested 750 consecutive invasive carcinomas for HER2 overexpression using both IHC and FISH techniques. He found that whereas the concordance rate between FISH and IHC (positive = 3+, negative = 1+, 0) was 98.7%. FISH was positive in 36% of specimens scored 2+ by IHC (Figure 2) which would have been scored negative by this technique. A pictorial comparison between the two testing methods is shown in figure 2[25].
Hoang et al [26] also reported a high concordance rate for HER2 positivity between IHC score 3+ and FISH (89%). However, there was a low interobserver reproducibility in separating 2+ from 3+cases. This data suggests that IHC is a useful initial test and cases scoring 2+ should be considered for FISH testing.
Mass from Genetech [27] recently presented the concordance rates between FISH and the clinical trial assay (CTA), a immunohistochemical technique in 623 samples randomly selected from the two pivotal Herceptin trials. FISH positivity was observed in 4.2%, 6.7%, 23.9% and 89.3% of CTA 0, 1+, 2+ and 3+, respectively.
Conflicting results regarding the best antibody to use in determining the IHC status of HER2 were presented by Falo et al [28]and Bartlett et al [29], respectively. The former found that monoclonal antibody CB11 was more reliable than the Dako polyclonal antibody compared against FISH, whereas Bartlett reported a higher accuracy for the polyclonal antibody (87.4%) compared with the monoclonal (83.8%). Gancberg et al [30] studied the sensitivity of three frequently used antibodies and found that the monoclonal antibody TAB250 had the lowest misclassification rate against FISH in 160 breast cancer specimens.
In view of such conflicting results and the significant inter-observer variation IHC should be performed in standardised reference laboratories with a large caseload. In the United Kingdom, all laboratories performing IHC as a predictive test must participate in an external quality assurance scheme and guidelines recommend that they should be performing at least 250 assays per year (100 for Her2 FISH testing. Alternatively, FISH testing could be performed as the gold standard. The latter option may need to be introduced gradually until the technique and required expertise are established in breast cancer centres.
FISH has been described as not only being both more accurate in determining HER2 status but also is a better predictor of prognosis and response to Herceptin. In addition, DNA is more stable than protein, interpretation is easier and there is less inter-operator error. FISH however is both more labour intensive and expensive.
For these reasons in the United Kingdom, a two-phase testing regimen exists for assessing HER2 status. Initial IHC testing of 0 or 1+ is reported as negative with 3+ being reported as positive. A score of 2+ is reported as intermediate with these cases being referred for FISH to establish a definitive diagnosis.
The clinical applications of Her2 testing
Does HER2 Overexpression Predict Adjuvant Tamoxifen Failure in Patients With Breast Cancer?
Tamoxifen is a selective oestrogen receptor (ER) modulator and has been proven to reduce relapse, death rates and risk of contralateral breast cancer and as such is probably the first truly targeted therapy for treating breast cancer [31]. Tamoxifen failure therefore is an area that generates intense research.
Both experimental and clinical evidence have indicated that the HER2 pathway interacts with the ER pathway with retrospective clinical studies suggesting an inverse relationship between ER and HER-2 expression. The proportion of patients with ER/HER-2 +ve breast tumours is approximately 9%.
Preclinical Evidence
Experimental evidence has shown that oestrogen-dependent MCF7 cells that over express HER2 are rendered tamoxifen resistant and have reduced numbers of ER [32, 33]. The promoter of the HER-2 gene also contains an oestrogen response element which suppresses HER-2 expression on response to oestrogen and is overexpressed with tamoxifen [34, 35]. Transfection of MCF-7 cells with the HER-2 coding region has also been shown to render them tamoxifen resistant [36]. For these reasons, the HER2 pathway has been investigated as a potential contributor towards Tamoxifen resistance and HER2 has been proposed as a potential marker of Tamoxifen sensitivity.
It appears that the mechanism for this resistance may rest in feedback through joint downstream signalling mechanisms. It has already been described that HER-2 transfected MCF-7 cells are tamoxifen resistant but HER-2 overexpression/stimulation leads to ER downregulation, increased phosphorylation and also increased transcriptional activation [37–39]. In addition, HER-2 downregulation appears to shut down HER-2 initiated MAP kinase pathways and makes other ER-linked apoptotic pathways dominant [40]. Supporting this, blocking MAP kinase in these HER-2 +, ER + cells restores their sensitivity to tamoxifen. Another feature of interest is that MEKK1, a downstream Her-2 signalling mediator activates ER and potentiates the agonist effect of tamoxifen [41]. In this way, HER-2 positivity may act to convert tamoxifen from anantagonist to agonist in breast cancer cells.
Clinical Evidence
Many clinical studies have found an association between HER2 overexpression and Tamoxifen failure in metastatic breast cancer [42–45] and also a reduced response duration and survival duration in those treataed with adjuvant hormonal therapies. The GUN trial [46], revealed that HER-2 expression not only predicted tamoxifen resistance but also showed a worse outcome on tamoxifen compared to those who were untreated (possibly because of the heightened agonist action). Recently a meta-analysis of seven studies[47] concluded that metastatic breast cancer overexpressing HER2 was very likely to be resistant to Tamoxifen (odds ratio of disease progression, 2.46). Recent work presented at the San Antonio breast cancer conference however did not find such an association. In a large cohort of stage 2 breast cancer patients randomised to recieve either 2 years of tamoxifen or nothing, no difference in tamoxifen efficacy was seen in Her2 -ve patients when compared to those who were Her 2 +ve.
It still remains controversial however as to whether adjuvant Tamoxifen has beneficial or detrimental effects in early breast cancer over expressing HER2 [48, 48–51]. Five of six studies so far have shown that treating patients who are HER2 positive with adjuvant Tamoxifen does not have a beneficial effect, and Bianco et al [52] have even reported a detrimental effect.
Among patients receiving adjuvant Tamoxifen, a combined analysis [53] was undertaken of studies with analysable data [48, 51, 52, 54] using the number of patients and the length of median follow up as a weighting factor for the relative risk of relapse or death per study. This revealed that HER2 over expression was associated with an increased the risk of relapse/death by 75% (RR = 1.75). Such results strongly suggest that over expression of HER2 predicts reduced response to adjuvant tamoxifen in patients with EBC, but does not exclude benefit in patients with HER2 and ER/PgR-positive tumours.
Available clinical evidence, although limited, suggests that the response to aromatase inhibitors in HER-2 positive breast cancers is superior to tamoxifen. In randomised studies of ER +ve HER-2 +ve patients treated with either Tamoxifen or Letrazole, there was a good response in the letrazole arm compared to a negligible response with Tamoxifen [55, 56]. It is postulated that this may be due to the fact that aromatase inhibitors effectively reduce the amount of active oestrogen rendering the ER monomeric and inactive, whilst the agonist activity of Tamoxifen can still be activated by MEKK1.
The role of Herceptin in early breast cancer
Herceptin [31] is a chimeric IgG monoclonal antibody (95% human, 5% murine) developed from the murine 4D5 antibody. It targets the external moiety of the Her2 receptor and prevents activation of the protein (possibly by preventing dimerisation) thereby preventing proliferation of breast cancer cells overexpressing Her2.
Pre-clinical Trials
In pre-clinical studies, anti HER2 MABs have been shown to inhibit the growth of HER2 over expressing tumour cells.
Harwerth et al, [57] looked at the effects of HER2-specific MAB administration on the tumorigenic growth of human HER2 transformed NIH3T3 cells implanted into athymic nude mice. Two antibodies (FWP51 and FSP77) inhibited the onset of tumour growth, and led to retardation of growth of established tumours. They were also effective in the treatment of transformed tumours established from SKOV3 cells [58]. Furthermore, the authors observed that the combination of the two antibodies, which react with two distinct regions of the HER2 receptor, was more effective than treatment with either MAB alone.
Hudziak et al, [59] showed that a MAB directed against the extracellular domain of p185 HER2 specifically inhibited the growth of breast tumour derived cells over expressing the HER2 gene product. They also showed that resistance to the cytotoxic effect of tumour necrosis factor alpha (a consequence of HER2 overexpression) was significantly reduced in the presence of the antibody.
Clinical trials
The initial trials regarding the use of Herceptin were concentrated on it's role in the treatment of metastatic breast cancer with anthracyclines, paclitaxel and doxetaxel. We will not be elaborating on this further but instead will concentrate on the evidence for it's use in EBC.
In the neoadjuvant setting, a preliminary small randomised trial showed an excellent pathological CR rate (67%) when Herceptin was used with a number of chemotherapeutic agents (particularly paclitaxel) followed by combination anthracycline treatment when compared with standard chemotherapy alone (25%) [60]. Following these remarkable results, 4 major trials were started recruiting 13,000 patients to assess the role of Herceptin further. Each trial used Herceptin for 1 year either in combination with or following chemotherapy and each has shown a recurrence risk reduction of approximately 50%. The two USA based trials (NSABBP B-31 and NCCTG N9831) combined their data showing benefit when Herceptin was used with paclitaxel following 4 courses of anthracycline [61]. The HERA trial showed similar results when Herceptin was used alone following standard chemotherapeutic regimens with a 46% recurrence risk reduction, 51% distant recurrence risk reduction and a 24% reduction in mortality (not significant), which was seen across all subsets [62]. Recent subgroup analysis has shown this relapse reduction to be independent of the nodal status or hormone receptor profile even in those patients with a relatively low risk of relapse. The BCIRG 006 trial showed similar benefit with docetaxel following 4 courses of anthracyclines [63]. This trial also showed benefit with a novel non-anthracycline schedule using upfront herceptin with docetaxel and carboplatin which would avoid anthracycline related cardiotoxicity. This latter schedule however is probably only as effective as anthracyclines in the 65% of patients whose tumours do not overexpress topo-isomerase 2, a key target of anthrocycline chemotherapy.
Maturation of these trials has also shown a significant survival benefit at 2 years followup of 34% (HERA) which is mirrored by the two American trials.
Although these results are undoubtedly very exciting it should be remembered that the followup is still relatively short. The optimal duration of Herceptin treatment is also subject of considerable debate. A 2 year extended treatment arm of the HERA trial is currently underway with results expected whilst a small Finnish trial has suggested a mere 9 week upfront course of Herceptin may have the same benefit as the larger trials [64].
The role of herceptin with adjuvant hormonal therapy
It has already been established that herceptin is an extremely useful adjuvant therapy in the HER-2 +ve, ER/PR -ve patient and also that it predicts for tamoxifen resistance. It would therefore seem reasonable to expect that administering Herceptin plus Tamoxifen to ER+ve HER-2 positive patients may overcome this Tamoxifen resistance. This has been proved preclinically using the parent antibody of Herceptin mAb 4D5 [36]. Several other cell culture studies have revealed greater growth inhibition using a combination of Herceptin and Tamoxifen on ER+ve/HER-2+ve cell lines when compared with each agent in isolation [36, 65] and have also showed that this is only the case with strongly ER+ve cell lines [66]. Several studies are currently underway investigating this phenomenon further.
The cost of Herceptin
Herceptin is not without complications with mild to moderate adverse effects occuring in approximately 50% of patients. The most common adverse reactions are related to the initial infusion particularly fever, chills, pain, vomiting and headache [24, 24, 67], and the most serious adverse reaction is class III/IV cardiac dysfunction. The NSABBP B-31 trial showed an increase in cardiotoxicity of 3.3% in the Herceptin vs the control arm, and the HERA trial produced a 1.7% increase in cardiac failure [68].
Cobleigh et al [69, 70] reported cardiac dysfunction in 10 patients (4.7%) nine of whom had received anthracycline therapy. There was one cardiac-related mortality. Such data suggest that the combination of anthracycline and herceptin should be avoided and the left ventricular ejection fraction should be measured in patients at risk. Herceptin-related cardiac dysfunction varies in severity and should be treated with standard medical therapy (diuretics, glycosides, ACE inhibitors, etc) and discontinuation of herceptin therapy should be considered when the risks outweigh the benefits.
Other adverse reactions include hypersensitivity reactions and anaphylaxis (rare), pulmonary events including dyspnoea, bronchospasm and ARDS (rare), haematological toxicity (leucopenia, thrombocytopenia and anaemia) and hepatic and renal toxicity. Many of these side effects are believed to be related to the 5% murine component located at the FAB end of the antibody. There have been no reports so far of the development of measurable antibodies to Herceptin inpatients who received the recommended dose.
Herceptin should be avoided in pregnancy as the teratogenic risk is as yet unknown and should also be avoided in breastfeeding and for 6 months afterwards.
Financially, treatment with herceptin and a taxane will cost approximately £20,000 per patient assuming that the treatment is stopped ater 18 weeks which does not include costs associated with testing, and investigation and treatment of cardiac morbidity.
Conclusion
Herceptin has emerged as the single most important treatment for breast cancer in both the metastatic and neoadjuvant settings, since the emergence of Tamoxifen. It is particularly exciting since it represents the first truly effective targeted treatment to molecular abnormalities in tumour cells. Although the trials are of relatively short followup duration, the treatment is associated with rare but significant side effects and the treatment is not without cost, Herceptin has undoubtedly made a real difference for those 25–30% of patients who overexpress the Her2/neu oncogene. Table 2 summarises the current place for Herceptin in early breast cancer.
In the future the role of combined Herceptin and hormonal therapies will hopefully become more evident which may further benefit this group of patients.
References
Nicholson S, Wright C, Sainsbury JR, Halcrow P, Kelly P, Angus B, Farndon JR, Harris AL: Epidermal growth factor receptor (EGFr) as a marker for poor prognosis in node-negative breast cancer patients: neu and tamoxifen failure. J Steroid Biochem Mol Biol. 37 (6): 811-4. 10.1016/0960-0760(90)90424-J. 1990 Dec 20
Bacus SS, Gudkov AV, Esteva FJ, Yarden Y: Expression of erbB receptors and their ligands in breast cancer: implications to biological behavior and therapeutic response. Breast Dis. 2000, 11: 63-75.
Bargmann CI, Hung MC, Weinberg RA: The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 319 (6050): 226-30. 10.1038/319226a0. 1986 Jan 16
Schechter AL, Hung MC, Vaidyanathan L, Weinberg RA, Yang-Feng TL, Francke U, Ullrich A, Coussens L: The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 229 (4717): 976-8. 10.1126/science.2992090. 1985 Sep 6
Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, Saito T, Toyoshima K: Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 319 (6050): 230-4. 10.1038/319230a0. 1986 Jan 16
Burden S, Yarden Y: Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997, 18 (6): 847-55. 10.1016/S0896-6273(00)80324-4.
Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Raatzkin BJ, Sela M, Yarden Y: Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 15 (10): 2452-67. 1996 May 15
di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA: erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 237 (4811): 178-82. 10.1126/science.2885917. 1987 Jul 10
di Fiore PP, Segatto O, Taylor WG, Aaronson SA, Pierce JH: EGF receptor and erbB-2 tyrosine kinase domains confer cell specificity for mitogenic signaling. Science. 248 (4951): 79-83. 10.1126/science.2181668. 1990 Apr 6
Hudziak RM, Schlessinger J, Ullrich A: Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci USA. 1987, 84 (20): 7159-63. 10.1073/pnas.84.20.7159.
Lonardo F, Di ME, King CR, Pierce JH, Segatto O, Aaronson SA, di Fiore PP: The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol. 1990, 2 (11): 992-1003.
Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T: The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 232 (4758): 1644-6. 10.1126/science.3012781. 1986 Jun 27
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235 (4785): 177-82. 10.1126/science.3798106. 1987 Jan 9
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Lein WJ, Stuart SG, Udove J, Ullrich A: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 244 (4905): 707-12. 10.1126/science.2470152. 1989 May 12
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235 (4785): 177-82. 10.1126/science.3798106. 1987 Jan 9
Stancovski I, Peles E, Ben LR, Lemprecht R, Kelman Z, Goldman-Michael R, Hurwitz E, Bacus S, Sela M, Yarden Y: Signal transduction by the neu/erbB-2 receptor: a potential target for anti-tumor therapy. J Steroid Biochem Mol Biol. 1992, 43 (1–3): 95-103. 10.1016/0960-0760(92)90192-L.
Giai M, Roagna R, Ponzone R, De BM, Dati C, Sismondi P: Prognostic and predictive relevance of c-erbB-2 and ras expression in node positive and negative breast cancer. Anticancer Res. 1994, 14 (3B): 1441-50.
Guerin M, Barrois M, Terrier MJ, Spielmann M, Riou G: Overexpression of either c-myc or c-erbB-2/neu proto-oncogenes in human breast carcinomas: correlation with poor prognosis. Oncogene Res. 1988, 3 (1): 21-31.
Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, Osbourne CK, Torney DC, McGuire WL: Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992, 23 (9): 974-9. 10.1016/0046-8177(92)90257-4.
Liu E, Thor A, He M, Barcos M, Ljung BM, Benz C: The HER2 (c-erbB-2) oncogene is frequently amplified in in situ carcinomas of the breast. Oncogene. 1992, 7 (5): 1027-32.
Mokbel K, Hassanally D: From HER2 to herceptin. Curr Med Res Opin. 2001, 17 (1): 51-9. 10.1185/03007990152005360.
Masood S, Bui MM: Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci. 2000, 30 (3): 259-65.
Niehans GA, Singleton TP, Dykoski D, Kiang DT: Stability of HER-2/neu expression over time and at multiple metastatic sites. JNatl Cancer Inst. 85 (15): 1230-5. 10.1093/jnci/85.15.1230. 1993 Aug 4
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999, 17 (9): 2639-48.
Ridolfi RL, Jamehdor MR, Arber JM: HER-2/neu testing in breast carcinoma: a combined immunohistochemical and fluorescence in situ hybridization approach. Mod Pathol. 2000, 13 (8): 866-73. 10.1038/modpathol.3880154.
Hoang MP, Sahin AA, Ordonez NG, Sneige N: HER-2/neu gene amplification compared with HER-2/neu protein overexpression and interobserver reproducibility in invasive breast carcinoma. Am J Clin Pathol. 2000, 113 (6): 852-9. 10.1309/VACP-VLQA-G9DX-VUDF.
Mass RD: Concordance between the clinical trial assay (TA) and fluorescence in situ hybridisation (FISH). Eur J Cancer. Edited by: Sanders C, Kasian C. 2000, 36: S52-S53.
Falo c: Her-2/neu determination in breast carcinoma. Comparison of two IHC methods in relation to FISH. Eur Journal Cancer. Edited by: Figueras A, Moreno B. 2000, 36: s52-
Bartlett JMS: IHC vs FISH for assessing Her-2 overexpression in breast cancer. Eur J Cancer. Edited by: Going JJ, Mallon EA. 2000, s52-
Gancberg D, Lespagnard L, Rouas G, Paesmans M, Piccart M, Di Leo A, Nogaret JM, Hertens D, Verhest A, Larsimont D: Sensitivity of HER-2/neu antibodies in archival tissue samples of invasive breast carcinomas. Correlation with oncogene amplification in 160 cases. Am J Clin Pathol. 2000, 113 (5): 675-82. 10.1309/0F58-0GRX-FK4R-A6VA.
Jones A: Combining trastuzumab (Herceptin) with hormonal therapy in breast cancer: what can be expected and why?. Ann Oncol. 2003, 14 (12): 1697-704. 10.1093/annonc/mdg483.
Read LD, Keith D, Slamon DJ, Katzenellenbogen BS: Hormonal modulation of HER-2/neu protooncogene messenger ribonucleic acid and p185 protein expression in human breast cancer cell lines. Cancer Res. 50 (13): 3947-51. 1990 Jul 1
Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ: HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 10 (12): 2435-46. 1995 Jun 15
Antoniotti S, Maggiora P, Dati C, De BM: Tamoxifen up-regulates c-erbB-2 expression in oestrogen-responsive breast cancer cells in vitro. Eur J Cancer. 1992, 28 (2–3): 318-21. 10.1016/S0959-8049(05)80045-0.
Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ, et al: HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 10 (12): 2435-46. 1995 Jun 15
Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osbourne CK: Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. BreastCancer Res Treat. 1992, 24 (2): 85-95. 10.1007/BF01961241.
Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ: HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 10 (12): 2435-46. 1995 Jun 15
Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P: Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 270 (5241): 1491-4. 10.1126/science.270.5241.1491. 1995 Dec 1
Bunone G, Briand PA, Miksicek RJ, Picard D: Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15 (9): 2174-83. 1996 May 1
Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH: Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 97 (3): 306-12. 10.1002/ijc.1614. 2002 Jan 20
Lee H, Jiang F, Wang Q, Nicosia SV, Yang J, Su B, Bai W: MEKK1 activation of human estrogen receptor alpha and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol Endocrinol. 2000, 14 (11): 1882-96. 10.1210/me.14.11.1882.
Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DB, Miller WR: Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 63 (19): 6523-31. 2003 Oct 1
Nicholson S, Wright C, Sainsbury JR, Halcrow P, Kelly P, Angus B, Farndon JR, Harris AL: Epidermal growth factor receptor (EGFr) as a marker for poor prognosis in node-negative breast cancer patients: neu and tamoxifen failure. J Steroid Biochem Mol Biol. 37 (6): 811-4. 10.1016/0960-0760(90)90424-J. 1990 Dec 20
Wright C, Nicholson S, Angus B, Sainsbury JR, Farndon J, Cairns J, Harris AL, Horne CH: Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992, 65 (1): 118-21.
Houston SJ, Plunkett TA, Barnes DM, Smith P, Rubens RD, Miles DW: Overexpression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. Br J Cancer. 1999, 79 (7–8): 1220-6. 10.1038/sj.bjc.6690196.
Carlomagno C, Orditura M, Pepe S, De Vita F, Romano C, Ciardiello F, Ferrara C, Martinelli E, Bianco R, Aurilio G, D'Agostino D, Tortora D, Catalanao G, De Placido S: Capecitabine plus weekly oxaliplatin in gastrointestinal tumors: a phase I study. Am J Clin Oncol. 2006, 29 (1): 85-9. 10.1097/01.coc.0000195087.24930.e7.
De Laurentiis : A meta analysis of the interaction between HER-2 and and the response to endocrine therapy (ET) in metastatic breat cancer (MBC). Proc ASCO. 2000, 19: Abstract 301-Ref Type: Generic
Tetu B, Brisson J: Prognostic significance of HER-2/neu oncoprotein expression in node-positive breast cancer. The influence of the pattern of immunostaining and adjuvant therapy. Cancer. 73 (9): 2359-65. 1994 May 1
Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J: Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998, 16 (2): 462-9.
Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A'Hern R: Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006, 17 (5): 818-26. 10.1093/annonc/mdl016.
Borg A, Baldetorp B, Ferno M, Killander D, Olsson H, Ryden S, Sigurdsson H: ERBB2 amplification is associated with tamoxifen resistance in steroid-receptor positive breast cancer. Cancer Lett. 81 (2): 137-44. 10.1016/0304-3835(94)90194-5. 1994 Jun 30
Bianco AR: Her-2 overexpression predicts adjuvant tamoxifen failure for early breast cancer (EBC): complete data at 20 yeard of the Naples GUN randomised trial. Proc ASCO. Edited by: De Laurentiis m, Carlomango C. 2000, 19: 289-Ref Type: Generic
Hu JC, Mokbel K: Does c-erbB2/HER2 overexpression predict adjuvant tamoxifen failure in patients with early breast cancer?. Eur J Surg Oncol. 2001, 27 (4): 335-7. 10.1053/ejso.2000.1078.
Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J: Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998, 16 (2): 462-9.
Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, Williams WR, Evans DB, Dugan M, Brady C, Wuebe-Fehling E, Borgs M: Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 19 (18): 3808-16. 2001 Sep 15
Ali SM: Predictive factors and response to letrazole vs taamoxifen. Breast Cancer Res. Edited by: Leitzel K, Demers L. 2002, 76: s32-Ref Type: Generic
Harwerth IM, Wels W, Schlegel J, Muller M, Hynes NE: Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993, 68 (6): 1140-5.
Harwerth IM, Wels W, Schlegel J, Muller M, Hynes NE: Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993, 68 (6): 1140-5.
Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A: p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989, 9 (3): 1165-72.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN: Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 23 (16): 3676-85. 10.1200/JCO.2005.07.032. 2005 Jun 1
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RG, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 353 (16): 1673-84. 10.1056/NEJMoa052122. 2005 Oct 20
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomsson C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 353 (16): 1659-72. 10.1056/NEJMoa052306. 2005 Oct 20
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Wolter J, Pegram M, Fleming T, Eiermann W, Baselga J, Norton L: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 344 (11): 783-92. 10.1056/NEJM200103153441101. 2001 Mar 15
Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utrainen T, Kokko R, Hemminki A, Tarkkenen M, Turpeenniemi-Hujanen T, Jyrkkio S, Flander M, Helle L, Ingalsuo S, Johannson K, Jaaskelainen AS, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, leinonen M, Elonaa I, Isola J: Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 354 (8): 809-20. 10.1056/NEJMoa053028. 2006 Feb 23
Witters LM, Kumar R, Chinchilli VM, Lipton A: Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat. 1997, 42 (1): 1-5. 10.1023/A:1005798224288.
Kunisue H, Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, Doihara H, Shimizu N, Snoo H: Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer. 2000, 82 (1): 46-51. 10.1054/bjoc.1999.0875.
Vogel C, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ: First-line, single-agent Herceptin(trastuzumab) in metastatic breast cancer: a preliminary report. Eur J Cancer. 2001, 37 (Suppl 1): S25-S29. 10.1016/S0959-8049(00)00405-6.
Tan-Chiu E, Yothers G, Romond E, Geyer CE, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark M, Bryant J: Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 23 (31): 7811-9. 10.1200/JCO.2005.02.4091. 2005 Nov 1
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999, 17 (9): 2639-48.
Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A'Hern R, Sainsbury R, Baum M: Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006, 17 (5): 818-26. 10.1093/annonc/mdl016.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Subramanian, A., Mokbel, K. The role of Herceptin in early breast cancer. Int Semin Surg Oncol 5, 9 (2008). https://doi.org/10.1186/1477-7800-5-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7800-5-9