Abstract
Background
Linoleic 18:2 (n-6) and α-linolenic 18:3 (n-3) essential fatty acids and long-chain polyunsaturated fatty acids (LC-PUFA) are essential nutrients for growth and neonatal development. Consumption of preformed n-3 LC-PUFA has been shown to increase gestational duration and to decrease the incidence of premature birth in human studies. This study evaluated the association of essential fatty acids and LC-PUFA in breast milk on the growth of premature children (weight, height and head circumference).
Study design
Thirty-seven premature infants with a gestational age of 37 weeks or less were followed until 6 months of gestational age, adjusted for prematurity. The milk from mothers, weight, height and head circumference measures of children were collected during the follow up. The breast milk fatty acids were quantified by gas-liquid chromatography.
Results
Our results showed that total n-3 PUFA was positively associated with weight gain (p = 0.05), height (p = 0.04) and body mass index (BMI) of children (p = 0.05). Our results also indicate that both linoleic acid and total essential fatty acids were positively associated with BMI and head circumference, whereas oleic acid was positively associated only with head circumference.
Conclusion
These results suggest that the n-3 PUFA composition of milk may be associated with weight gain and growth. Considering the advantages of n-3 LC-PUFA consumption on infant growth and visual function and its association with reduced incidence of premature birth, dietitians should advise pregnant women to increase their intake of foods high in n-3 LC-PUFA.
Similar content being viewed by others
Introduction
The n-3 polyunsaturated fatty acids, also known as omega 3 fatty acids, are fatty acids with the first double bound at the third position from the methyl end. They occur in the diet as α-linolenic acid (ALA, C18:3n-3) from vegetable sources and nuts, and as very-long-chain 'marine' n-3 polyunsaturated fatty acid (LC-PUFA) from fish and othern seafood. The main forms of marine n-3 LC-PUFA are eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3) [1]. Typical intake in Western populations is 1–2 g per day for ALA and 0–0.4 g per day for marine n-3 LC-PUFA [2]. The importance of ALA as a dietetic precursor to EPA and DHA in the human body has also been reported [3]; however, various studies have indicated that the conversion of ALA to functional forms of n-3 LC-PUFA, such as DHA and EPA, is not adequate human beings [2, 4, 5]. Because the placenta lacks desaturase activity and fetal enzyme activity in uterus is very limited, the fetus depends on placental LC-PUFA transfer [6].
Over the past few decades, n-3 LC-PUFAs have been shown to be essential for neurodevelopment [7–10]. These essential fatty acids, including DHA, α-linolenic acid, and other fatty acids of the n-3 series, are indispensable structural components of cellular membranes and are essential for cerebral and retinal growth, especially in the first two years after birth [11–13]. The deposition of DHA in the retina and in the cerebral cortex occur especially during the third trimester of gestation and in the first six months of extrauterine life; therefore, both the last three months of gestation and first few months after birth are particularly vulnerable to developmental deficits, if DHA is limiting [10, 14].
The premature babies, in particular, born during the last trimester of pregnancy have been shown to have low levels of DHA in the blood; this has been correlated with their abnormal eye and brain functions as measured by electroretinogram, cortical visual-evoked potential, and behavioral testing of visual acuity [15, 16]
In the other hand, consumption of preformed n-3 LC-PUFA has been shown to increase the duration of gestation and to decrease the incidence of premature birth in both human and animal studies [2, 17]. Thus, n-3 deficiency may enhance the risk of premature delivery. Therefore, adequate supplementation of n-3 LC-PUFA becomes essential during the prenatal period and is especially important for pre-mature children [18, 19]. The premature baby may be particularly at risk for deficiency of essential long chain fatty acids for the following reasons: 1) neonates and pre-mature children have less adipose tissue, and are therefore more prone to fatty acid deficiencies [13, 20]; 2) LC-PUFAs are deposited in considerable quantities during the last trimester of gestation for the growth of the brain and the retina, and premature children are not born with these deposits [3, 14]; and 3) the metabolism of the fatty acids in premature infants is still immature. The LC-PUFAS, DHA and AA can be synthesized from ALA and LA, although the activity of conversion is low in humans [2, 4, 5]. The extent of this conversion of ALA in premature children is not precisely known and is, at best, very limited. [3, 6, 21]
Although many studies have shown that n-3 LC-PUFAs are beneficial to infant development, the effects of maternal levels of these essential fatty acids on the growth of premature infants are not completely understood. Studies investigating the association of n-3 PUFAs in human milk are scarce. The objective of this study is to investigate associations between human milk levels of n-3 LC-PUFA, including essential fatty acids and long chain polyunsaturated fatty acids, and the growth of premature children.
Materials and methods
Process of Selecting the Population
Male and female children born prematurely (gestational age less than 37 weeks) in the Department of Neonatology of the Institute Fernandes Figueira – IFF/FIOCRUZ, Rio de Janeiro, Brazil were selected for this study The study was initiated only after written consent of the parent or guardian, and parents were fully informed of the study goals, methods, risks, and benefits before consent was obtained. Both the project and the consent form were approved by the Ethics Committee for Research in Humans of the Institute Fernandes Figueira/Oswaldo Cruz Foundation (IFF/FIOCRUZ, Rio de Janeiro, Brazil) before the study began and its conduction was in accordance with the Declaration of Helsinki. The children underwent ambulatory monitoring until they reached six months of corrected gestational age for prematurity (age of birth in gestational weeks less than 40 weeks–where the gravidic cycle is complete; full pregnancy).
The gestational age (GA) was determined from the information collected from the mothers or, in cases where the information was not precise, an estimate of the GA was made. This estimate was based on the date of the last menstrual period (LMP), which was either reported by the mother or identified by ultrasound at the 20th week of gestation. In cases where there was a lack of information, we evaluated the GA by using the method of New Ballard [22].
Initially, 215 premature children were selected. Of these, 47 were excluded because they presented a form of pathology (carriers of syndromes or genetic birth defects, alterations in the gastrointestinal tract or the respiratory tract, renal dysfunction, neurological disorders, the presence of congenital infections such as rubella, citomegalia, toxoplasmosis, and herpes, or children of mothers carrying the HIV virus), or because they were receiving artificial milk. In the first month of follow-up, there were 3 deaths and 12 losses due to mothers who never returned for the follow-up of their baby. Of the 153 premature children, 50 had no collection of milk at birth (colostrum) because the mothers had initial difficulties in the collection of milk due to the following reasons: refusal (did not cooperate with the study), had no milk before hospital discharge, were already drawing milk for the milk bank, declined due to pain, or did not schedule a time to meet with the researcher upon their return visit to the hospital during the first few days after delivery. Of the 103 children for whom their mother's milk was collected, 38 had milk collected at baseline only (colostrum), and 65 children had milk collected at baseline (colostrum) as well as in the follow-up period. Thus, the study population includes 65 children who were breastfed for at least 1 month of follow-up. Of these 65 children, 37 were randomly selected for analysis of fatty acids in the maternal milk.
For a type I error of 0.05 and a type II error of 0.20 with a standard deviation of 1 unit and a difference of 1 unit, the required sample size would be 32 children. Therefore, for the estimated number of 37 children, it is possible to observe changes to the standard deviations up to 1.53 units at baseline when the samples are independent.
Collection of Data
The weight, height, and head circumference were obtained by anthropometry once a month at the outpatient visit. The body weight of premature infants was measured in a calibrated digital balance (Fillizola) when the babies were naked. Their length was measured by placing the premature infants in a horizontal decubitus, where they were measured from the crown to the heel. The head circumference was obtained by using an measuring tape at the supra-orbital ridges and at the largest frontoccipital diameter.
The nursing team collected the breast milk during the monthly outpatient visit. One milliliter of milk was collected from each mother and then deposited in identified ependorffs. The milk samples were frozen at -70°C for later analysis.
Fatty acid analysis
The fatty acids in the breast milk were evaluated by gas chromatography. The extraction, saponification and lipid methylation were performed in duplicate from 200 μl of milk, according to the method of Lepage and Roy [23], which recommends treatment with 2 ml methanol: toluene 4:1 (v/v) solution and 200 μl of acetyl chloride added under light agitation. The methyl esters were quantified by gas-liquid chromatography using a Perkin Elmer Autosystem XL chromatograph equipped with an ionized flame detector (FID) and a software turbochrom. The fatty acids were separated on a 100 × 0.25 mm × 0.20 μm capillary column SP 2560 (Supelco, USA). The gas chromatographic conditions were the same as those described elsewhere [24] The esters were identified by comparison with their retention time with known standards (Sigma, Supelco). The results were expressed as the percentage of total fatty acids.
Statistical analysis
The selected premature infants were analyzed for changes in body weight, height and head circumference, and these data were correlated with the concentrations of various fatty acids in milk that could be related to growth. The chromatographic analysis was performed on the milk collected from their mothers shortly after delivery (colostrum), and a second assessment of milk was collected between post-partum days 35 and 42 from the mother of the same child (mature milk).
The databases were constructed using software EPI INFO [25]. The follow-up data from the cohort of premature infants were analyzed using the proc mixed procedure of the SAS statistical package (Statistic Analysis System), version 8.0 (SAS Institute, Cary, NC, USA) [26, 27].
For each one of the outcomes: weight, height, body mass index (BMI) and head circumference, regression models included as independent variables the difference between mature milk – colostrum of fatty acids, time and an interaction factor (differences in fatty acids * time). Therefore, if BMI increased during follow-up and this change was related to fatty acids the variable both time and the interaction factor will be statistically significant.
The difference of values of fatty acids for the two collection times for milk (mature milk and colostrum) was categorized in below the median and above the median allowing plotting two curves for comparisons as in the figures 1 and 2. The difference of values of fatty acids for the two time points is positive when the value for mature milk was higher than that of colostrum, so values above the median also represent increasing levels of fatty acids and vice versa.
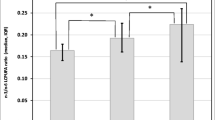
Estimated values for body mass index (Kg/m2) (BMI). Model included the difference of linoleic fatty acid (A), and total essential fatty acid (B) between of mature milk and colostrum, time of follow-up (time) and the interaction difference of fatty acids *time. A – linoleic fatty acid. B – total essential fatty acid.
Estimated values for head circumference (cm). Model included the difference of linoleic fatty acid (A), and total essential fatty acid (B), oleic fatty acid (C) between of mature milk and colostrum, time of follow-up (time) and the interaction difference of fatty acids *time. A – linoleic fatty acid. B – total essential fatty acid. C – oleic fatty acid.
Results
Table 1 shows the anthropometric measures according to the time of follow-up, initiated at birth (0) and up to six months (1 to 6) of life. The nutritional assessment data indicate that female children had an average birth weight of 1790 g and an average height of 43 cm. The average male height and weight at birth were 44.8 cm and 2135 g, respectively. Values of head circumference are also presented in Table 1, but these measures were not registered at birth or during the first month of life. All children exceeded the 2500 g recommended by WHO/OMS at two months of life.
The differences between the fatty acids for the two collection times for milk (mature milk and colostrum) were evaluated in relation to anthropometric variables in the multivariate regression analysis. Gestational age and birth weight, variables known to influence growth, were initially included in the models, but because they did not change the associations of fatty acids and growth they were excluded. Therefore, the models that are presented in Tables 2, 3, 4 and 5 include only the fatty acid differences, time and the interaction between time and the fatty acids.
The regression coefficient (β) for weight in grams (g) indicated variations positive over time, which was statistically significant for all fatty acids. The total n-3 PUFA was positively associated with weight gain (p = 0.05). The variable of interaction between time and the fatty acid difference estimates the rate of change of weight in relation to different fatty acids, was statistically significant and negative for acid DHA, linoleic, total LC-PUFA, and total essential fatty acids (Table 2).
Table 3 shows estimated values of the coefficients of the fatty acid variables for height in centimeters (cm). Based on these figures, we observed that the total n-3 PUFA was positively associated with a gain in height (p = 0.04). The rate of change of growth (difference of fatty acid*time), in relation to all fatty acids was not statistically significant.
Our results showed a positive and statistically significant association (p = 0.05) of total n-3-PUFA with BMI. The rate of change of body mass index (BMI) was negative and statistically significant for the DHA, linoleic acid, total LC-PUFA and total essential fatty acids. (Table 4).
The estimated values of the coefficients for head circumference are in Table 5. The rate of change of the head circumference was statistically significant in relation to oleic acid, total saturated fatty acid, EPA, total n-3 PUFA, linoleic acid, total n-6 PUFA and total essential fatty acid. (Table 5).
The estimated values of BMI for values above and bellow the differences in fatty acids based on the regression model which included also time of follow-up and the interaction variable between time and categorized value for the difference of fatty acids are represented in Figures 1 for linoleic acid (Figure 1A), that shows that the smaller reduction in milk of linoleic acid levels was associated with greater increase in BMI. Figure 1B shows the estimate change in BMI according to the variation for total essential fatty acids (α-linolenic and linoleic). The estimated parameters are in Table 4.
Figures 2 shows the estimated values for head circumference, which estimated parameters are in Table 5. We observed that the smaller reduction in milk of linoleic acid levels, the greater the increase in head circumference at follow-up (Figure 2A). In Figures 2B and 2C indicates that there is a greater head circumference increase in children at the follow-up when there is a smaller reduction in the levels of total essential fatty acids (α-linolenic and linoleic) and oleic acid levels in milk.
Discussion
In this study, we analyzed the effect of fatty acids on anthropometric variables in premature infants, where the results showed that the total amount of n-3 PUFA found in mother's milk was positively associated with weight gain (p = 0.05), gain of height (p = 0.04) and BMI of premature children (p = 0.05) (Table 2, 3 and 4). These data confirm the importance of n-3 PUFA in the development and growth of premature children.
In fact, the n-3 PUFA, including DHA, α-linolenic acid and other fatty acids of the n-3 series, are essential structural components for cell membranes and are important for the growth of the brain and retina, especially in the first two years after birth [12, 13]. However, an important aspect to be emphasized in the results of this study was the influence of these n-3 PUFAs not only on weight, but also on height and, consequently, on the BMI in the first months of life of a premature child. This effect can be explained by the fact that n-3 PUFAs are mainly involved in cell growth and multiplication [13], thus exerting a positive effect on weight and height of children. Olsen et al. [28] also demonstrated an association between increased consumption of dietary n-3 PUFA by pregnant women with increasing birth weight of their children. A study involving 8,729 pregnant Danish women found that low fish consumption was a strong risk factor for preterm delivery and low birth weight [29]. Therefore, there is a need for greater maternal intake of n-3 PUFAs during the intra-uterine phase and during the first months of an infant's life [10] because the mother has the fundamental role in the transfer of these fatty acids through the placenta and breast milk [19]. Because n-3 PUFA can influence a wide variety of biological functions, including being essential for the growth and function of the human body, it is important that expectant mothers meet the nutritional recommendations for the consumption these fatty acids in their diets [19].
Because of the inherent problems with assessing diet and maintaining appropriate food composition tables, many investigators are now assessing dietary intake of PUFAs using biomarkers; in general, the concentrations of PUFAs in breast milk reflect the maternal intake of the previous day [30]. Innis [31] found a positive correlation between the content of DHA in milk with visual and language development in breast-fed infants, while a recent Norwegian study found that children of mothers who received supplementation with cod liver oil during pregnancy had an improved mental processing score at four years of age [16]. Assessing the n-3 PUFA levels in the milk of mothers of premature babies, we observed that there was no significant variation in the total amount of n-3 PUFA found in the colostrum compared to mature milk. We hypothesized that the presence of n-3 PUFA in milk may be associated with weight gain and structural development in children.
Although the rate of change in BMI was negative in relation to linoleic acid and total essential fatty acids, our results indicate that both linoleic acid and total essential fatty acid are associated with increased BMI in premature children. In the model adjusted by time of follow-up and the interaction variable between time and categorized value for the difference of fatty acids (difference of fatty acids*time), it can be seen that the smaller reductions in the linoleic acid levels (Figure 1A) and total essential fatty acid (Figure 1B), the greater the increase in BMI during the follow-up period. Similar results were observed for head circumference in relation to linoleic acid, total essential fatty acid and oleic acid (Table 5); the higher the levels of linoleic acid (Figure 2A), oleic acid (Figure 2C) and total essential fatty acids (Figure 2B) in milk, the greater the increase in head circumference during the follow-up. Within the diversity of fatty acids, we can say that not only the LC-PUFAs, but also their metabolic precursors, the essential fatty acids (linoleic and linolenic), are transferred through breast milk to the child and may also exert a positive effect on the health of the child by encouraging early brain development and growth, as was reflected by the increase in BMI. Essential fatty acids also have an important role during the intra-uterine phase, extending the length of gestation and thereby influencing birth weight [3]. The unquestionable necessity of essential fatty acids is demonstrated by these results, so the type of acid that accumulates in fetal tissue can determine weight gain, height gain and increases in head circumference.
In summary, this study demonstrates the influence of maternal lipid consumption during lactation or breastfeeding in the growth of premature infants. It is clear that dietary polyunsaturated fatty acids are transferred into human breast milk and, subsequently, to the child. The results of this study point to the influence of maternal nutrition during pregnancy and lactation on infant growth in the first months of life, reinforcing the need for maternal nutritional education about appropriate consumption of n-3 PUFA and essential fatty acids to support healthy infant growth [9].
In conclusion, we found that the quality of dietary lipids offered through breast milk during the first months of life is a critically important determinant in the of growth premature infants.
References
Ruxton CH, Reed SC, Simpson MJ, Millington KJ: The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. 2007, 20: 275-85. 10.1111/j.1365-277X.2007.00770.x
Perez MA, Hansen RA, Harris MA, Allen KGD: Dietary docosahexaenoic acid alters pregnant rat reproductive tissue prostaglandin and matrix metalloproteinase production. J Nutr Biochem. 2006, 17: 446-53. 10.1016/j.jnutbio.2005.10.003
Allen KGD, Harris MA: The Role of n-3 Fatty Acids in Gestation and Parturition. Exp Biol Med. 2001, 226: 498-506.
Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N: Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J Lipid Res. 2001, 42: 1257-65.
Brenna JT: Efficiency of conversion of α-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002, 5: 127-32. 10.1097/00075197-200203000-00002
Hanebutt FL, Demmelmair H, Schiessl B, Larque' E, Koletzko B: Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clinical Nutrition. 2008, 27: 685-693. 10.1016/j.clnu.2008.05.010
Smit EN, Oelen EA, Seerar E, Muskiet FAJ, Boersma ER: Breastmilk docosahexaenoic acid (DHA) correlates with DHA status of malnourished infants. Arch Dis Child. 2000, 6: 493-4. 10.1136/adc.82.6.493.
Uauy R, Valenzuela A: Marine Oils: The Health Benefits of n-3 Fatty Acids. Nutrition. 2000, 16: 680-4. 10.1016/S0899-9007(00)00326-9
Vidailhet M: Oméga 3: une situation de carence chez le jeune enfant?. Arch Pediatr. 2007, 14: 116-23. 10.1016/j.arcped.2006.09.020
Sala-Vila A, Castellote AI, López-Sabater MC: The intramolecular position of docosahexaenoic acid in the triacylglycerol sources used for pediatric nutrition has a minimal effect on its metabolic use. Nutrition Research. 2008, 28: 131-36. 10.1016/j.nutres.2007.11.007
Innis SM, Adamkin DH, Hall RT, Kalhan SC, Lair C, Lim M, Stevens DC, Twist PF, Diersen-Schade DA, Harris CL, Merkel KL, Hansen JW: Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J Pediatr. 2002, 140: 547-54. 10.1067/mpd.2002.123282
Das UN: Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition. 2003, 19: 62-5. 10.1016/S0899-9007(02)00852-3
Innis SM: Essential Fatty Acid Transfer and Fetal Development. Placenta. 2005, 26: S70-S5. 10.1016/j.placenta.2005.01.005
Gaete GM, Atalah SE, Araya AJ: Efect de la suplementación de la dieta de la madre durante la lactancia con ácidos grasos omega 3 en la composición de los lípidos de la leche. Rev Chil Pediatr. 2002, 73: 239-47.
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE: Essential fatty acids in visual and brain development. Lipids. 2001, 36: 885-95. 10.1007/s11745-001-0798-1
Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA: Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003, 111: e39-44. 10.1542/peds.111.1.e39
Allen KGD, Harris MA: The role of n-3 fatty acids in gestation and parturition. Exp Biol Med. 2001, 226: 498-506.
Gil A, Ramirez M, Gil M: Role of Long-chain polyunsaturated fatty acids in infant nutrition. Eur J Clin Nutr. 2003, 57: S31-S4. 10.1038/sj.ejcn.1601810
Innis SM: Trans fatty intakes during pregnancy, infancy and early childhood. Atheroscler Suppl. 2006, 7: 17-20. 10.1016/j.atherosclerosissup.2006.04.005
Hornstra G: Essential fatty acids in mothers and their neonates. Am J Clin Nutr. 2000, 71: 1262S-9S.
Larqué E, Zamora S, Gil A: Dietary trans fatty acids in early life: a review. Early Hum Dev. 2001, 65: S31-S41. 10.1016/S0378-3782(01)00201-8
Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R: New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991, 119: 417-23. 10.1016/S0022-3476(05)82056-6
Lepage G, Roy CC: Direct transesterification of all classes of lipid in on-step reaction. J Lipid Res. 1986, 27: 114-20.
Tinoco SMB, Sichieri R, Setta CL, Moura AS, Tavares do Carmo MG: Trans fatty acids from milk of Brazilian mothers of premature infants. J Paediatr Child Health. 2008, 44: 50-6.
Dean AG, Dean JA, Coulombier D, Burton AH, Brendel KA, Smith DC, Burton AH, Dicker RC, Sullivan K, Fagan RF, Arner TG: Epi-info version 6: a word processing database, and statistics program for epidemiology on microcomputers. 1994, Atlanta: Center for Disease Control and Prevention Geórgia, USA,
Littell RC, Milliken GA, Stroup WW, Wolfinger RD: SAS system for mixed models. 1996, SAS Institute Inc,
Garrett FM: Statistical Analysis System Selection SAS Documentation for Bios 226. ab: Applied longitudinal Data Analysis. 2000, SAS Institute Inc. SAS Campus Drive, Cary, NC, USA, 3,
Olsen F, Grandjean P, Weihe P, Vider T: Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose dependent relationship. J Epidemiol Community Health. 1993, 47: 436-440. 10.1136/jech.47.6.436
Olsen SF, Secher NJ: Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. BMJ. 2002, 23: 447-10.1136/bmj.324.7335.447.
Craig-Schmidt MC: World-wide consumption of trans fatty acids. Atheroscler Suppl. 2006, 7: 1-4. 10.1016/j.atherosclerosissup.2006.04.001
Innis SM: Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003, 143: S1-8.
Acknowledgements
We acknowledge the nurses of the Department of Neonatology of the Institute Fernandes Figueira – IFF/FIOCRUZ, Rio de Janeiro, Brazil who have assisted in the collection of breast milk and we also thank the mothers who have donated breast milk. The present work was in part supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SMB made substantial contributions to conception and design, acquisition of data and also the analysis and interpretation of data. RS participated in the design of the study and performed the statistical analysis and helped to draft the manuscript. CLS participated in the fatty acid analysis. ASM made substantial contributions to conception and design of the study and interpretation of the data and coordination to draft the manuscript. MGTC made substantial contributions to the conception and design, analysis and interpretation of the data and coordination to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tinoco, S.M.B., Sichieri, R., Setta, C.L. et al. n-3 polyunsaturated fatty acids in milk is associate to weight gain and growth in premature infants. Lipids Health Dis 8, 23 (2009). https://doi.org/10.1186/1476-511X-8-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-8-23