Abstract
Background
Our previous study demonstrated that the A-allele of the single nucleotide polymorphism (SNP) rs34623097 located in the upstream region of the β2 adrenergic receptor gene (ADRB2) is significantly associated with risk for obesity in Oceanic populations.
Methods
To investigate whether the ADRB2 polymorphisms explain part of the individual differences in lipid mobilization, energy expenditure and glycogen breakdown, the associations of 10 ADRB2 SNPs with total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglyceride levels were examined in 128 adults in Tonga.
Results
A multiple linear regression analysis adjusted for age, sex, and body mass index revealed that rs34623097 was significantly associated with triglyceride levels (P-value = 0.037). A copy of the rs34623097-A allele increased serum triglyceride levels by 70.1 mg/dL (0.791 mmol/L). None of the ADRB2 SNPs showed a significant association with total-cholesterol, high-density lipoprotein cholesterol, or low-density lipoprotein cholesterol.
Conclusions
In a Tongan population, a SNP located in the upstream region of ADRB2 is associated with triglyceride levels independent of body mass index.
Similar content being viewed by others
Background
The β2 adrenergic receptor (ADRB2), a class of G protein-coupled receptor for catecholamines, plays an important role in regulating energy expenditure and lipolysis in adipose tissue. Therefore, polymorphisms in the ADRB2 gene (OMIM*109690) may explain part of the individual differences in lipid profiles. The 27Glu allele at the Glu27Gln polymorphism of ADRB2 has been significantly associated with increased triglyceride levels [1–6], although there is a conflicting report that demonstrated a significant increase in triglyceride levels in individuals with the Gln/Glngenotype as compared to those with the Glu/Glugenotype [7].
Most studies have examined the association of three non synonymous ADRB2 SNPs, rs1042711 (5’LC-Arg19Cys in the 5’ upstream region), rs1042713 (Gly16Arg) and rs1042714 (Glu27Gln), with lipid profiles (i.e., total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol and triglyceride levels). However, ourrecent study showed that rs34623097 located in the upstream region of ADRB2 is more strongly associated with obesity than non synonymous SNPs in Oceanic populations. A functional analysis suggested that rs34623097-A, a risk allele for obesity, reduces the transcriptional activity of ADRB2 as compared with rs34623097-G [8]. The aim of the present study is to further explore the association of ADRB2 SNPs including rs34623097 with lipid profiles independent of body mass index (BMI) in adult Tongan subjects.
Results
Subject characteristics
A total of 128 unrelated subjects (40 men and 88 women) were recruited for the present study. The clinical and laboratory parameters of participants are summarized in Table 1. The median values of total cholesterol and triglycerides in Tongan male subjects exceeded the desirable ranges of less than 200 mg/dL for total cholesterol and less than 150 mg/dL for triglycerides. Age was significantly associated with total cholesterol and LDL cholesterol; sex and BMI were significantly associated with HDL cholesterol and triglycerides (Table 2).
Association of ADRB2 polymorphisms with lipid profiles
Two samples for rs11959427, three samples for rs1042713, and two samples for rs1042714 were not successfully genotyped by a molecular biology-based technique. To avoid a reduction in statistical power, these genotypes were imputed by using the MACH software [9]. Then we manually checked the imputed genotypes based on the LD structure (Figure 1), and concluded that the imputed genotypes seemed to be valid. The following analyses were therefore performed for the data including the imputed genotypes. No significant deviation from Hardy-Weinberg equilibrium was observed for the 10 ADRB2 SNPs.
We adjusted the crude effect of each ADRB2 SNP by taking into account age, sex and BMI, since these variables are significantly associated with lipid traits (Table 2). A multiple linear regression analysis adjusted for age, sex, and BMI indicated that rs34623097 was significantly associated with triglycerides in Tongan subjects (Table 3). A copy of the rs34623097-A allele, a risk allele for obesity in Oceanic populations [8], increased serum triglyceride levels by 70.1 mg/dL (0.791 mmol/L). No other significant associations were detected. It is noted that the derived allele, regardless of the allele frequency, was considered when determining the direction of association in Table 3.
In this study, besides nine tag SNPs (rs17778257, rs34623097, rs2895795, rs2053044, rs11959427, rs1042711, rs1042713, rs1042714, and rs1042720) of ADRB2, the rs1042719 SNP was further evaluated becausers 1042720, being in LD with rs1042719 in the Oceanic populations [8], showed a small P-value for triglyceride (P-value = 0.059; Table 3); however, the rs1042719 SNP was also not significantly associated with the level of triglycerides (P-value = 0.057).
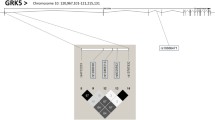
To assess the possibility that the association of rs34623097 with triglycerides is caused by nearby SNPs being in LD with rs34623097, an additional multiple regression analysis was further performed for 7 SNPs imputed by the MACH software [9] by using the genotype data of HapMap East Asian populations (JPT + CHB) as the reference panel. No SNPs other than rs34623097 showed a significant association with triglycerides (Figure 2).
Regional association plot. The genotypes of SNPs around the ADRB2 gene in Tongan subjects were imputed by the MACH software by using the genotype data of HapMap JPT and CHB samples as the reference panel. The association P-values for 10 genotyped and 7 imputed SNPs are plotted as diamonds and circles, respectively. The r2 between the SNP and rs34623097 is colored as a scale from low (blue) to high (red). In the genomic region, there is no protein-coding gene other than ADRB2.
Discussion
The present results indicate that rs34623097-A is associated with increased serum triglyceride levels. Our previous luciferase reporter assay demonstrated that rs34623097-A reduces the transcriptional activity of ADRB2 as compared with rs34623097-G, and an electrophoretic mobility shift assay suggested that rs34623097 modulates the binding affinity with nuclear factors [8]. Taken together, these results indicate that the reduced expression of ADRB2 caused by rs34623097-A on adipocytes might contribute, in part, to increased serum triglyceride levels. One possible mechanism is that the activation of β adrenergic receptors such as ADRB2 expressed in adipocytes leads to the breakdown of triglycerides stored in adipocytes and the release of free fatty acids and glycerol [10–12]. Thus, the decreased lipolytic function in adipocytes is due to the lower expression of ADRB2 that would result in the accumulation of triglycerides within adipocytes. If triglycerides accumulate in adipocytes, the cellular uptake of the major component of triglycerides (i.e., free fatty acids) by adipocytes would be reduced. This mechanism would reduce the lipolysis of circulating lipoprotein-triglycerides. Accordingly, the level of serum triglycerides would be increased in individuals with rs34623097-A.
To the best of our knowledge, this study is the first to report that rs34623097 is a major ADRB2 polymorphism that influences the serum triglyceride level, although the possibility that the lack of association of the other ADRB2 polymorphisms with lipid profiles comes from the small sample size (n = 128) should not be excluded. Previous studies have revealed that the 27Glu allele (rs1042714-G) of ADRB2 significantly increases the serum triglyceride levels [1–6]. In the present study, 27Glu also showed the same tendency (i.e., the β coefficient of 27Gln, an alternative allele at Glu27Gln, was a negative value in Table 3). This tendency is because rs34623097-A, which is significantly associated with increased triglycerides, is in positive LD with 27Glu in the Tongan subjects (D’ = 1 and r2 = 0.62). The rs34623097-A allele is observed mainly in Asians and Pacific Islanders and is in LD with 27Glu [8]. However, rs34623097-A had never previously been examined in the association studies on lipid traits including triglycerides. The significant association of 27Glu found in previous studies may merely reflect the LD with rs34623097-A. Further studies are required to clarify this issue in various ethnic groups.
Subjects and methods
Subjects
A total of 128 healthy adult subjects (18 years old or older) were recruited from Nuku’alofa, Tonga. Patients with diabetes and subjects who had any treatment known to interfere with metabolic syndrome-related parameters were excluded. A blood sample was collected from each subject after obtaining a written consent to participate in the study. This study was approved by the National Health Ethics & Research Committee of Tonga and the Research Ethics Committee of the Faculty of Medicine, University of Tsukuba.
Anthropometric measurements
Anthropomorphic phenotypes were directly measured in field settings. Measurements were taken of subjects dressed in light clothing. Body height was measured to the nearest 1 mm by using a field anthropometer (GPM, Zurich, Switzerland), and weight was recorded to the nearest 0.1 kg by using a portable digital scale (Tanita model BC-518, Tokyo, Japan). BMI was calculated by dividing the weight in kg by the height in meters squared.
Methods
Blood samples were obtained on the morning following a 12-hour fast. Serum lipids including total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were measured at SRL Inc. (Tokyo, Japan) by using standard laboratory protocols.
Genotyping
Genomic DNA was extracted from peripheral blood by using a QIAamp Blood Kit (Qiagen, Hilden, Germany). Nine tag SNPs (rs17778257, rs34623097, rs2895795, rs2053044, rs11959427, rs1042711, rs1042713, rs1042714, and rs1042720) of ADRB2, which were in LD (r2 > 0.8) with the other ADRB2 SNPs in the Oceanic populations, were genotyped in our previous study [8]. The rs1042719 SNP was genotyped by using a TaqMan SNP genotyping assay in the present study because rs1042720, being in LD with rs1042719, showed not a significant but a small P-value in the association analyses of triglycerides in a Tongan population.
Statistical analysis
Associations of age, sex, and BMI with total-cholesterol, HDL-cholesterol, LDL-cholesterol or triglyceride levels were assessed by a multiple regression analysis. The genotypes of rs11959427, rs1042713, and rs1042714 were not determined by a molecular biology-based technique for some subjects; instead, their genotypes were imputed by the MACH software [9]. Deviation of genotype frequencies from Hardy-Weinberg equilibrium was examined by chi-square test. Pairwise linkage disequilibrium (LD) parameters, D’ and r2, were estimated by using Haploview software [13]. The association of each polymorphism with total cholesterol, HDL cholesterol, LDL cholesterol or triglyceride levels was assessed by a multiple regression analysis adjusted for age, sex, and BMI. In the regression analysis, the number of copies of a derived allele at each SNP was used as an independent variable (i.e., homozygotes of an ancestral allele, homozygotes of a derived allele, and heterozygotes were coded as 0, 2, and 1, respectively). The genotypes of SNPs with minor allele frequency (MAF) of ≥ 0.05 spanning a 200 kb genomic region containing the entire ADRB2 gene were retrieved from the HapMap JPT (Japanese in Tokyo, Japan) and CHB (Han Chinese in Beijing, China) populations [14, 15]. Furthermore, genomic DNA samples from 43 JPT and 45 CHB subjects were obtained from the Coriell Cell Repository and subjected to rs34623097 genotyping [8]. By using the genotype data of JPT and CHB subjects as a reference, the genotypes of Tongan subjects were imputed by the MACH software [9]. The imputed genotypes of SNPs showing an Rsq value (a measure which estimates the squared correlation between imputed and true genotypes) of more than 0.5 were used for the association test. Accordingly, seven imputed SNPs were further subjected to single-point association analysis. P-values less than 0.05 were regarded as statistically significant.
Conclusions
In a Tongan population, the rs34623097-A allele at a SNP located in the upstream region of ADRB2 is significantly associated with increased serum triglyceride levels independent of BMI.
References
Ehrenborg E, Skogsberg J, Ruotolo G, Large V, Eriksson P, Arner P, Hamsten A: The Q/E27 polymorphism in the beta(2)-adrenoceptor gene is associated with increased body weight and dyslipoproteinaemia involving triglyceride-rich lipoproteins. J Intern Med. 2000, 247 (6): 651-656. 10.1046/j.1365-2796.2000.00669.x
Iwamoto N, Ogawa Y, Kajihara S, Hisatomi A, Yasutake T, Yoshimura T, Mizuta T, Hara T, Ozaki I, Yamamoto K: Gln27Glu beta2-adrenergic receptor variant is associated with hypertriglyceridemia and the development of fatty liver. Clinica chimica acta; international journal of clinical chemistry. 2001, 314 (1–2): 85-91.
Ishiyama-Shigemoto S, Yamada K, Yuan X, Ichikawa F, Nonaka K: Association of polymorphisms in the beta2-adrenergic receptor gene with obesity, hypertriglyceridaemia, and diabetes mellitus. Diabetologia. 1999, 42 (1): 98-101. 10.1007/s001250051120
Macho-Azcarate T, Marti A, Gonzalez A, Martinez JA, Ibanez J: Gln27Glu polymorphism in the beta2 adrenergic receptor gene and lipid metabolism during exercise in obese women. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002, 26 (11): 1434-1441. 10.1038/sj.ijo.0802129.
Isaza C, Henao J, Ramirez E, Cuesta F, Cacabelos R: Polymorphic variants of the beta2-adrenergic receptor (ADRB2) gene and ADRB2-related propanolol-induced dyslipidemia in the Colombian population. Methods Find Exp Clin Pharmacol. 2005, 27 (4): 237-244. 10.1358/mf.2005.27.4.893582
Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, Nishigaki Y: Association of genetic variants with chronic kidney disease in individuals with different lipid profiles. Int J Mol Med. 2009, 24 (2): 233-246.
Petrone A, Zavarella S, Iacobellis G, Zampetti S, Vania A, Di Pietro S, Galgani A, Leonetti F, Di Mario U, Buzzetti R: Association of beta2 adrenergic receptor polymorphisms and related haplotypes with triglyceride and LDL-cholesterol levels. European journal of human genetics: EJHG. 2006, 14 (1): 94-100.
Naka I, Hikami K, Nakayama K, Koga M, Nishida N, Kimura R, Furusawa T, Natsuhara K, Yamauchi T, Nakazawa M: A functional SNP upstream of the beta-2 adrenergic receptor gene (ADRB2) is associated with obesity in Oceanic populations. Int J Obes (Lond). in press.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR: MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010, 34 (8): 816-834. 10.1002/gepi.20533
Haffner CA, Kendall MJ, Maxwell S, Hughes B: The lipolytic effect of beta 1- and beta 2-adrenoceptor activation in healthy human volunteers. Br J Clin Pharmacol. 1993, 35 (1): 35-39. 10.1111/j.1365-2125.1993.tb05667.x
Large V, Arner P: Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab. 1998, 24 (5): 409-418.
Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M: In situ assessment of the role of the beta 1-, beta 2- and beta 3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol. 1996, 117 (5): 907-913. 10.1111/j.1476-5381.1996.tb15279.x
Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005, 21 (2): 263-265. 10.1093/bioinformatics/bth457
Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM: A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007, 449 (7164): 851-861. 10.1038/nature06258
The International HapMap Consortium: A haplotype map of the human genome. Nature. 2005, 437 (7063): 1299-1320. 10.1038/nature04226
Acknowledgements
We sincerely thank the people of Tonga for their kind approval and support of our research. We are grateful to Dr. Taniela Palu (Diabetes Clinic, Kingdom of Tonga) and Dr. Viliami Tangi (Minister of Health, Kingdom of Tonga). This work was partly supported by a KAKENHI (22370084 and 25291103) Grant-in-Aid for Scientific Research (B) and a Grant-in-Aid for JSPS Fellows (24.5) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IN and JO performed statistical analyses and wrote the manuscript. IN and RK extracted DNA. IN performed genotyping. JO, RK, TI, and YM measured anthropomorphic phenotypes and collected blood samples. TI and YM contributed acquisition of lipid profile data. IN, JO, RK, TI, and YM participated in the design and coordination of the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Naka, I., Ohashi, J., Kimura, R. et al. Association of ADRB2 polymorphism with triglyceride levels in Tongans. Lipids Health Dis 12, 110 (2013). https://doi.org/10.1186/1476-511X-12-110
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-12-110