Abstract
Background
Myc is a well known driver of lymphomagenesis, and Myc-activating chromosomal translocation is the recognized hallmark of Burkitt lymphoma, an aggressive form of non-Hodgkin's lymphoma. We developed a model that mimics this translocation event by inserting a mouse Myc cDNA gene into the immunoglobulin heavy chain locus, just upstream of the intronic Eμ enhancer. These mice, designated iMycEμ, readily develop B-cell lymphoma. To study the mechanism of Myc-induced lymphoma, we analyzed signaling pathways in lymphoblastic B-cell lymphomas (LBLs) from iMycEμ mice, and an LBL-derived cell line, iMycEμ-1.
Results
Nuclear factor-κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) were constitutively activated in iMycEμ mice, not only in LBLs but also in the splenic B-lymphocytes of young animals months before tumors developed. Moreover, inhibition of either transcription factor in iMycEμ-1 cells suppressed growth and caused apoptosis, and the abrogation of NF-κB activity reduced DNA binding by both STAT3 and Myc, as well as Myc expression. Inhibition of STAT3 signaling eliminated the activity of both NF-κB and Myc, and resulted in a corresponding decrease in the level of Myc. Thus, in iMycEμ-1 cells NF-κB and STAT3 are co-dependent and can both regulate Myc. Consistent with this, NF-κB and phosphorylated STAT3 were physically associated with one another. In addition, LBLs and iMycEμ-1 cells also showed constitutive AKT phosphorylation. Blocking AKT activation by inhibiting PI3K reduced iMycEμ-1 cell proliferation and caused apoptosis, via downregulation of NF-κB and STAT3 activity and a reduction of Myc levels. Co-treatment with NF-κB, STAT3 or/and PI3K inhibitors led to additive inhibition of iMycEμ-1 cell proliferation, suggesting that these signaling pathways converge.
Conclusions
Our findings support the notion that constitutive activation of NF-κB and STAT3 depends on upstream signaling through PI3K, and that this activation is important for cell survival and proliferation, as well as for maintaining the level of Myc. Together, these data implicate crosstalk among NF-κB, STAT3 and PI3K in the development of iMycEμ B-cell lymphomas.
Similar content being viewed by others
Background
Deregulated NF-κB activity plays a critical role in the survival and radiation resistance of tumor cells in a variety of human neoplasias including B cell lymphomas (BCLs) [1–5]. NF-κB comprises a family of transcription factors that control genes implicated in B-cell activation, proliferation and resistance to apoptosis [6]. Five known, structurally conserved members of the NF-κB/Rel family function as dimers in various combinations: p50, p52, p65 (Rel A), Rel B and c-Rel. Classic NF-κB, the p50 and p65 heterodimer, is an activator of gene transcription, whereas the p50/p50 homodimer both represses and activates the transcription of target genes [7]. NF-κB exists in an inactive form in the cytoplasm because of its interaction with the inhibitory protein, IκBα [8]. NF-κB activation is controlled by the IκB kinase (IKK) complex; after stimulation by cytokines and/or growth factors, IKK phosphorylates IκB, which results in its subsequent ubiquitination and proteasomal degradation. The degradation of IκB allows NF-κB to translocate to the nucleus, where it can activate or repress target genes [9]. NF-κB not only plays a role in the survival of neoplastic B cells, but is also critical for the development and survival of normal B cells [10].
Another family of transcription factors whose members are constitutively activated in many human tumors is the STAT family. These proteins can control various cellular events such as proliferation, differentiation and cell survival [11]. One member in particular, STAT3, has been shown to be constitutively activated in a number of human tumor cell lines and primary tumors, including several hematological malignancies [12, 13]. STAT3 can be activated by IL6, interferons, epidermal growth factor or leptin, through the activity of members of the receptor-associated Janus kinase (JAK) family, which comprises JAK1, JAK2, JAK3, or TYK2 [14–16]. JAKs phosphorylate STAT3 at tyrosine (Tyr)-705, leading to its dimerization and subsequent translocation to the nucleus where it activates target genes [17]. In addition, maximal transcriptional activation of STAT3 requires phosphorylation at serine (Ser)-727 in response to cytokine stimulation [18–20].
Yet another important pathway of signal transduction in B cells and B-cell neoplasms is one involving phosphatidyl inositol-3 kinase (PI3K) and AKT. Aberrant activation of this pathway is a common molecular alteration in human malignancies [21–25]. PI3K becomes activated by receptor tyrosine kinases or other cell-surface receptors, resulting in an elevation in the production of the membrane lipid phospho-inositol (3,4,5)P3 (PIP3) from phospho-inositol(4,5)P2 (PIP2). The level of PIP3 is negatively controlled by the phosphatase and tensin homolog (PTEN), which converts PIP3 back to PIP2. AKT binds PIP3 at the plasma membrane, and this leads to phosphorylation of AKT at Ser-473 in its regulatory domain. This activated form of AKT can then phosphorylate, and thereby regulate the function of, many cellular proteins that are involved in cell proliferation and survival, as well as in tumorigenesis and metastasis [26–30].
Although activation of NF-κB, STAT3 and/or the PI3K/AKT pathway in B cell neoplasms has been described [23–25], the mechanism by which these pathways contribute to the development of BCLs remains unclear, as do the circumstances under which this occurs. We recently developed the iMycEμ mouse, an experimental model system for studying Myc-driven neoplastic transformation of B cells. Previous studies have shown that, on a mixed background of segregating C57BL/6 and 129/SvJ alleles, the iMyc transgene causes the development of various B cell-derived lymphomas: lymphoblastic B-cell lymphomas (LBLs) in 50% of the mice; diffuse large B-cell lymphomas (DLBCLs) in 25% of the mice, and plasmacytomas (PCTs) in 20% of the mice [31]. In the study described here, we investigated the role of NF-κB, STAT3 and PI3K signaling in LBL, the most prevalent tumor type in the iMycEμ mice. We found that constitutive activation of NF-κB and STAT3 begins well before frank tumors develop, with co-activation of NF-κB and STAT3 playing a role in tumor maintenance, and activation of the PI3K/AKT pathway in the neoplastic B cells being responsible, in part, for the constitutive activation of NF-κB and STAT3. Inhibition of any one of these three pathways resulted in Myc downregulation, inhibited growth growth and promoted apoptosis in iMycEμ-LBL-derived cells. We report, for the first time, a physical association of NF-κB with STAT3 in B cells, and provide evidence for the convergence of PI3K, NF-κB and STAT3 signaling in Myc-driven lymphomagenesis.
Materials and methods
Tissues and cell lines
Primary LBL tumors from iMycEμ mice [31] and the LBL-derived cell line, iMycEμ-1 [32], were used in this study. WEHI 231, RAW 8.1, and NFS-1.0 C-1 cell lines were purchased from ATCC (Rockville, MD). All cell lines were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 200 mM L-glutamine, 50 mM 2-mercaptoethanol and antibiotics, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco-BRL, Rockville, MD), at 37°C in a humidified 5% CO2 incubator. Highly enriched (>95% pure) splenic B cells were isolated from C57BL/6 (BL6) or iMycEμ mice using CD45R (B220) microbeads and MACS® separation columns (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Control cultures were treated with phosphate-buffered saline (PBS) or DMSO where appropriate, and the final concentration never exceeded 0.3%.
Preparation of nuclear and cytosolic extracts
Pellets of 107 cells or powdered-frozen LBL samples were lysed with 400 μl of 10 mM KCl, 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM DTT, and 0.2 mM PMSF at 4°C for 10 minutes. The lysate was centrifuged for 5 minutes at 14,000 × g and supernatants were stored as cytosolic extract, at -70°C. The resulting pellet was re-suspended in 100 μl of ice-cold 20 mM HEPES (pH 7.9), 420 mM NaCl, 1.5 mM MgCl2, 20% (v/v) glycerol, 0.2 mM EDTA, 0.5 mM DTT, and 0.2 mM PMSF. After incubation at 4°C for 20 minutes, the lysate was centrifuged for 6 minutes at 14,000 × g, and the supernatant was stored as a nuclear extract, at -70°C. The concentration of cytosolic and nuclear extract was determined using a BCA kit (Bio-Rad, Richmond, CA).
Electrophoretic mobility shift assay (EMSA) and super-shift assay
The DNA-protein binding detection kit (Gibco-BRL) was used with modifications. In brief, DNA-binding reactions were carried out in a final volume of 25 μl of buffer containing 10 mM Tris (pH 7.5), 100 mM NaCl, 1 mM DTT, 1 mM EDTA, 4% (w/v) glycerol, 0.1 mg/ml sonicated salmon sperm DNA, 10 μg of nuclear extract, and oligonucleotides. Oligonucleotides containing consensus NF-κB (Promega, Madison, WI), STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA), or Myc-Max binding sites (Santa Cruz Biotechnology) were end-labeled to a specific activity of 105 CPM with γ-[32P]-ATP and T4-polynucleotide kinase, followed by purification on a Nick column (GE Healthcare, Piscataway, NJ). Reaction mixtures with radio-labeled oligonucleotides were incubated at room temperature for 20 minutes, and resolved on 6% non-denaturing polyacrylamide gels after addition of 2 μl bromophenol blue (0.1%). Gels were dried and subjected to autoradiography. For competition assays, 30-fold excess unlabeled oligonucleotides containing consensus or mutated NF-κB, STAT3 or Myc-Max binding sites, respectively, were added for 20 minutes at room temperature, after incubation with the radio-labeled oligonucleotides. For super-shift assays, 2 μg of antibody (Ab) was added for 20 minutes at room temperature after the initial incubation. Abs specific for p50 (sc-114X), p52 (sc-298X), p65 (sc-109X), RelB (sc-226X), c-Rel (sc-70X), Myc (sc-764X), SP-1 (sc-59X), STAT3 (sc-483X) or P-STAT3 (sc-8059X or sc-7993X) were purchased from Santa Cruz Biotechnology.
Reverse transcription polymerase chain reaction (RT-PCR)
Semi-quantitative RT-PCR was performed by extracting total RNA using TRIzol (Sigma-Aldrich, St. Louis, MO), and this was followed by double-stranded cDNA synthesis from 1 μg of total RNA, using the AMV reverse transcriptase kit (Roche, Indianapolis, IN). Thermal cycling conditions were as follows: 95°C for 5 minutes followed by 20, 25, 30, 35, or 40 cycles (depending on the target gene) of amplification at 57°C, 72°C, and 95°C, for 1 minute each. PCR products were resolved by electrophoresis on 1% agarose gels containing ethidium bromide. Primer sequences are as follows:
PTEN, forward 5'-GGCGGTGTCATAATGTCTCTCA-3'
reverse 5'-CCCATTTTCCACTTTTTCTGAGG-3'
β-actin, forward 5'-ATGGCATTGTTACCAACTGGGACG-3'
reverse 5'-CTCTTTGATGTCACGCACGATTTC-3'.
Whole-cell extracts and Western blotting
Whole-cell lysates were obtained by re-suspending pellets of 107 cells or powdered-frozen LBL samples in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/ml PMSF, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4°C for 30 minutes. Lysates were centrifuged for 6 minutes at 14 000 × g, and supernatants were stored at -70°C as a whole-cell extract. Total protein concentrations were determined by BCA (Bio-Rad). Western blotting was performed with 40 μg of total protein resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were probed with Abs against c-Myc (sc-764), PTEN (sc-7974), or IκBα (sc-847) from Santa Cruz Biotechnology, ERK1/2 (9102), P-ERK1/2 (4377), p38 (9212), P-p38 (9211), AKT (9272), P-AKT (9271), P-AKT (9275), p70S6K (9202), or P-p70S6K (9205) from Cell Signaling (Danvers, MA), α-tubulin (T6074) or β-actin (A5316) from Sigma-Aldrich. Proteins were visualized using horseradish peroxidase-conjugated secondary Ab (1:5000) and the ECL detection kit from Amersham (GE Healthcare). To confirm equal loading, membranes were stripped and re-probed using an Ab specific for α-tubulin or β-actin. Total cell extracts from UV-treated HeLa and NIH 3T3 cells were used as positive controls for P-ERK1/2 (sc-2221) and P-p38 (sc-2210), respectively (Santa Cruz Biotechnology). Total cell extract from insulin-treated MCF-7 cells was used as a positive control for P-p70S6K (9203) (Cell Signaling).
Proliferation assay
Proliferation was determined using the Cell Titer 96® MTS/PMS assay (Promega). Briefly, 3 × 104 cells were re-suspended in 100 μl growth medium and plated into 96-well plates (Costar, Cambridge, MA). After 20 hours at 37°C and 5% CO2, 20 μl of MTS/PMS solution was added to each well and cells were incubated for another 4 hours before the absorbance at 490 nm was measured on a Multiskan Spectrum (Thermo Scientific, Hudson, NH).
Apoptosis assay
Apoptosis was evaluated using the DNA fragmentation assay and fluorescence activated cell sorting (FACS)-based analysis of propidium iodide (PI), annexin V, and Caspase-3 reactivity. For DNA fragmentation, DNA was extracted using the Puregene kit (Gentra Systems, Minneapolis, MN) and resolved by electrophoresis on 1.0% agarose gels containing ethidium bromide. For the identification of cells with sub-G0/G1 DNA content, cells were resuspended in PI/Rnase buffer (BD Pharmingen, San Diego, CA) for 20 minutes at 37°C in the dark before FACS analysis. Annexin-V reactivity was determined by applying a phycoerythrin (PE)-labeled Ab (BD Pharmingen) to cells co-stained with 7-amino-actinomycin D (7-AAD). Activated caspase-3 was detected using a FITC- or PE-conjugated Ab (BD Pharmingen).
Pharmacological inhibitors
The following small-molecule inhibitors were used: LY294002 (LY) (Promega); Lactacystin (LC), PD98059 (PD), SB203580 (SB), and rapamycin (Rap) (Biomol, Plymouth Meeting, PA); WHI P-131 (WHI) and AG 490 (Biosource, Carlsbad, CA); and AEG 3482 (AEG) (Tocris, Ellisville, MO).
Co-Immunoprecipitation (Co-IP)
Co-IP was performed using the Universal Magnetic Co-IP kit according to the manufacturer's protocol (Active Motif, Carlsbad, CA). Briefly, 500 μg of nuclear extract was incubated with 5 μg of phosphorylated STAT3 (P-STAT3) Ab (sc-7993), NF-κB p50 Ab (sc-114), or Rabbit IgG control (sc-2345) for 3 hour at 4°C. 25 μl of Protein G Magnetic Beads were added to each tube and then incubated for 1 hour at 4°C. Immunoprecipitates were washed 4 times each with 500 μl wash buffer using a magnetic stand, after which the pellets were resuspended with 2 × reducing loading buffer. Western blot analysis was performed using a primary NF-κB p50 Ab (sc-114) or P-STAT3 Ab (sc-7993), respectively.
IL6 and IL10 assays
IL6 or IL10 expression was assesssed using the RayBio Mouse Cytokine Antibody Array III kit (RayBiotech, Norcross, GA) or the Mouse IL-6 or IL-10 Enzyme-Linked Immunosorbent Assay (ELISA) kit (eBioscience, San Diego, CA), according to the manufacturer's protocol. Samples tested were 107 splenic B or B220-negative cells from 2-month-old BL6 or iMycEμ mice, separated by CD45R (B220) microbeads and MACS® separation columns (Miltenyi Biotec).
Results
NF-κB and STAT3 are constitutively activated in B-cell lymphomas of iMycEμ mice
Both NF-κB and STAT3 are important for the proliferation and survival of normal B cells and several types of non-Hodgkin's lymphoma (NHL) [33–37]. We used EMSA to examine NF-κB and STAT3 activity in both iMycEμ-derived LBLs and the iMycEμ-1 cell line. All nine LBLs and the iMycEμ-1 cells showed abnormal activation of both NF-κB (Figure 1A) and STAT3 (Figure 1B) when compared to isolated splenic B cells from control C57BL/6 (BL6) mice.
NF-κB and STAT3 are constitutively activated in LBLs and iMycEμ-1 cells. (A and B) EMSA using an NF-κB-specific probe (A) and a STAT3-specific probe (B), respectively, showing constitutive DNA-binding by NF-κB and STAT3 in LBL tumors (left panel), iMycEμ-1 cells (right panel) and control (C) C57BL/6 splenic B cells. (C) EMSA competition assay (left panel) demonstrating specificity of NF-κB probe. Right panel shows a super-shift assay for NF-κB, using antibodies (Abs) specific for the denoted subunits. Asterisks denote non-specific bands that are covered by the super-shifted bands in lanes 2 and 6. (D) Competition (left panel) and super-shift (right panel) assays for STAT3. Competitor is an unlabelled oligonucleotide probe, and mutator is an unlabelled probe with a mutation that abrogates DNA-binding. P-STAT3 super-shift Abs are both specific for phosphorylated STAT3 at Tyr-705; Ab 1 is sc-7993X and Ab 2 is sc-8059X. SP1 and Myc Abs were used as negative controls. Arrowheads denote shifted bands. Images are representative, and image splicing was carried out only for the same experiment, the same gel and the same exposure times.
To ascertain the specificity and subunit composition of NF-κB, we conducted competition and super-shift assays on iMycEμ-1 cells. Incubation of nuclear extracts with 30-fold excess unlabelled competitor probe abolished the constitutive NF-κB activity, but incubation with unlabelled probes containing a mutation that disables NF-κB binding (mutator) did not (Figure 1C, left), indicating that the observed band was indeed NF-κB. Super-shift assays were carried out using antibodies (Ab) against NF-κB subunit p 50, p 52, p 65, Rel B, or c-Rel. As shown in the right panel of figure 1C, notable shifts were observed when antibodies against p50 (lane 2), p 65 (lane 4) or c-Rel (lane 6) were added. The p50 Ab shifted both NF-κB-specific bands to higher molecular-weight positions, whereas the p 65 and c-Rel antibodies shifted only the upper band. Neither the p 52 nore the RelB antibody produced any shift. These results indicate that the constitutively activated NF-κB in iMycEμ-1 cells is likely comprised of p 50/p 50 homodimers and/or p 50/p 65 and p 50/c-Rel heterodimers. That the observed shift involving p65 was less pronounced suggests that p 50/p 50 and p 50/c-Rel complexes predominate.
Competition and super-shift assays were also performed for STAT3. Incubation of nuclear extracts with competitor abrogated the constitutive STAT3 activity, whereas the addition of mutator did not (Figure 1D, left). Incubation with one Ab specific for STAT3 phosphorylated at Tyr-705 shifted the band to a higher molecular weight, and incubation with another Ab completely eliminated the STAT3 band (Figure 1D, right, lanes 2 and 3, respectively). These results show that the activated form of STAT3 is phosphorylated on Tyr-705. Myc Ab and SP1 Ab were used as negative controls and did not show any change.
Constitutive activation of NF-κB and STAT3 occurs early in iMycEμ mice
The use of mouse models offers a valuable opportunity to study early events that contribute to tumor development. To determine whether NF-κB and STAT3 activation occurred before tumors were present, we examined NF-κB and STAT3 activity in splenic B cells from tumor-free (premalignant) or tumor-bearing (malignant) iMycEμ mice, using splenomegaly and age as two independent indicators of tumor progression. As expected, NF-κB (Figure 2A) and STAT3 (Figure 2B) activity was increased in splenic B cells isolated from mice with malignant growths (i.e. mice with spleen masses of on average 750 mg) relative to that in splenic B cells from normal BL6 mice (i.e. spleen masses between 80-100 mg). However, splenic B cells from iMycEμ mice with no visible signs of malignancy and spleen masses between 80-150 mg, which were considered premalignant, also had abnormally high NF-κB and STAT3 activity. Similarly, splenic B cells from one to four month-old premalignant iMycEμ mice (as defined without regard to spleen weight) exhibited highly elevated NF-κB and STAT3 DNA-binding activity, at as early as one month of age, relative to splenic B cells from age-matched, normal BL6 mice (see additional file 1A and 1B). These data show that constitutive activation of both NF-κB and STAT3 occurs months before tumors are present, and at an early age, in iMycEμ mice [31].
Constitutive activation of NF-κB and STAT3 in iMycEμ mice occurs early. (A and B) EMSA showing elevated DNA-binding activity of NF-κB (A) and STAT3 (B), respectively, in both premalignant (average spleen weight 80-150 mg) and malignant (average spleen weight ~750 mg) splenic B220-positive B cells from iMycEμ and control BL6 (average spleen weight 80-100 mg) mice. (C) Western blot showing the level of Myc protein in control, premalignant and malignant splenic B cells, defined as above, and in iMycEμ-1 cells. β-actin was used as a loading control. (D and E) Cytokine array (Left panel) and ELISA (Right panel) for IL6 and IL10, showing the levels of IL6 and IL10 protein in splenic B cells (D) and in splenic B220-negative cells (E) from 2-month old control or iMycEμ mice. ImageQuant software was used to quantitate the results of cytokine array, and data are expressed as arbitrary densitometric units (AU) after normalization to positive controls (Pos). "Neg" denotes negative controls.
We also evaluated the level of Myc protein in splenic B cells of premalignant and malignant iMycEμ mice (as defined by spleen size), as well as in iMycEμ-1 cells (Figure 2C). It is widely accepted that the cellular level of Myc must remain exquisitely titrated to induce neoplastic development but avoid apoptosis [38]. Consistent with this, only a marginal elevation of Myc protein was repeatedly observed in premalignant iMycEμ B splenocytes. Myc protein was, however, dramatically elevated in malignant B cells and in iMycEμ-1 cells. Even though NF-κB and STAT3 are known to drive Myc expression [34, 38–41], constitutive activity of NF-κB and STAT3 is not sufficient to increase the level of Myc at premalignancy in iMycEμ B cells.
IL6 and IL10 are important cytokines that have been implicated in lymphomagenesis and are linked to NF-κB and STAT3 signaling through autocrine and/or paracrine loops [4, 14, 39]. We performed cytokine array and ELISA to examine whether elevated expression of IL6 and/or IL10 are involved in early activation of NF-κB and STAT3 in iMycEμ mice. As shown in Figure 2D, no significant difference was observed in the level of either IL6 or IL10 between the splenic B cells of BL6 and premalignant iMycEμ mice, suggesting that elevated levels of IL6 and IL10 are not responsible for elevated NF-κB or STAT3 activity through autocrine signaling. IL6 and IL10 expression was also nearly equivalent in splenic B220-negative cells from premalignant iMycEμ and control mice (Figure 2F), suggesting that IL6 and IL10 are not upregulated in the B-cell microenvironment. Additionally, we independently evaluated the levels of IL6 and IL10 in LBL tumors using RT-PCR, GEArray (SA Bioscience, Frederick, MD) and Affymetrix GeneChip Arrays (Affymatrix, Santa Clara, CA) (n = 11, 8 and 4, respectively). No elevation of IL6 and IL10 expression has been observed in these iMycEμ tumors compared to normal BL6 splenic B cells (data not shown). These data suggest that the overexpression of IL6 and IL10 does not occur as a response to elevated NF-κB or STAT3 activity, nor as a cause thereof, through either autocrine or paracrine signaling in iMycEμ mice.
Inhibition of NF-κB in iMycEμ-1 cells reduces cell proliferation, causes apoptosis, and downregulates STAT3 activity and Myc expression
To investigate the role of NF-κB in proliferation and survival, we cultured iMycEμ-1 cells in the presence of the NF-κB inhibitor, Lactacystin (LC). LC treatment for 24 hours inhibited growth of iMycEμ-1 cells in dose-dependent fashion, as measured by MTS (Figure 3A). DNA laddering indicated that LC also induced apoptosis (Figure 3D). By EMSA, we confirmed that 5 μM LC inhibited NF-κB activity (Figure 3C) by stabilizing IκB (Figure 3D). Notably, other NF-κB inhibitors, BAY-11 7085 or Helenin, which function by blocking IκB phosphorylation or preventing DNA-binding by NF-κB, respectively, had similar inhibitory effects on the proliferation of iMycEμ-1 cells (data not shown). We then examined whether inhibiting NF-κB altered STAT3 or Myc activity. As shown in Figure 3E and 3F, treatment with LC dramatically reduced the activity of both STAT3 and Myc. The reduction in Myc activity corresponded to a remarkable decrease in the level of Myc protein (Figure 3G). EMSA competition and super-shift assays were done as before, to demonstrate the specificity of Myc DNA-binding (see additional file 2). These data imply that NF-κB is necessary for the proliferation and survival of iMycEμ-1 cells, and to link NF-κB to the activities of STAT3 and Myc.
NF-κB inhibition suppresses growth, causes apoptosis and downregulates STAT3 and Myc activity in iMycEμ-1 cells. (A) MTS/PMS cell proliferation assay, after culture with the NF-kB inhibitor lactacystin (LC) at various concentrations as indicated. Data were normalized to vehicle control, and error bars represent the standard deviation from a representative experiment performed in triplicate. (B) Agarose gel showing DNA fragmentation in sample treated with LC, but not in PBS control. (C) EMSA showing reduced NF-κB DNA-binding after LC treatment. (D) Stabilization of IκB protein after treatment with LC, as determined by Western blotting. (E) Reduced STAT3 DNA-binding activity after NF-κB inhibition, as observed by EMSA. (F) EMSA showing that Myc DNA-binding activity is reduced after LC treatment. (G) Western blot showing that Myc protein levels are reduced after NF-κB inhibition. β-actin was used as a loading control for Western blots. All LC incubations were for 24 hours.
STAT3 is required for optimal proliferation and survival of iMycEμ-1 cells, and is linked to activation of NF-κB and Myc
STAT3 was also constitutively activated in iMycEμ LBLs, so we examined whether signaling through this transcription factor is important for the proliferation and survival of iMycEμ-1 cells. Cells were cultured in the presence of the potent JAK3/STAT3 specific inhibitor WHI P-131 (WHI), and this suppressed growth in a dose-dependent manner (Figure 4A) and ultimately led to apoptosis (Figure 4B) through abrogation of STAT3 activity (Figure 4C). Use of the potent JAK2/STAT3-specific inhibitor AG 490 resulted in similar inhibitory effects on the proliferation of iMycEμ-1 cells (data not shown). We then assessed whether STAT3 signaling had an effect on NF-κB and/or Myc activity. Inhibiting STAT3 severely reduced the DNA binding activity of both NF-κB (Figure 4D) and Myc (Figure 4E), and led to a reduction in Myc protein levels (Figure 4F). Like NF-κB, STAT3 appears to be necessary for the proliferation and survival of iMycEμ-1 cells. Thus STAT3 is reciprocally linked to NF-κB activity and has similar effects on Myc, a finding that intimates a co-dependency between NF-κB and STAT3 signaling.
STAT3 inhibition reduces growth, leads to apoptosis and downregulates NF-κB and Myc activity in iMycEμ-1 cells. (A) Dose-dependent suppression of proliferation (MTS/PMS) after culture with the STAT3 inhibitor WHI P-131 (WHI) at various concentrations, as indicated. Data were normalized to DMSO treatment controls, and error bars represent the standard deviation for a representative experiment performed in triplicate. (B) Agarose gel showing DNA fragmentation after treatment with WHI. (C, D and E) EMSA revealing reduced STAT3 (C), NF-κB (D) and Myc (E) DNA-binding, respectively, after WHI treatment. (F) Western blot showing a decrease in the level of Myc protein after STAT3 inhibition. β-actin was used as a loading control. All WHI incubations were for 24 hours.
NF-κB and phosphorylated STAT3 associate physically in iMycEμ-1 cells
Recent studies have shown that NF-κB and STAT3 physically associate with one another in several cell types [42–46]. Our findings indicate that constitutively activated NF-κB and STAT3 may cooperatively regulate each other. Thus, we investigated whether STAT3 and NF-κB are physically associated in iMycEμ-1 cells. Super-shift assays were performed with a STAT3-specific oligonucleotide probe and antibodies specific for p 50, p 65, or c-Rel NF-κB subunits. As shown in Figure 5A, our results showed a clear shift in DNA-bound STAT3 when a p 50 Ab was added (lane 2). Addition of a p 65 Ab (lane 3) or c-Rel Ab (lane 4) led to a slight decrease in band intensity (~1/3 of control). This suggests that p65 and c-Rel may be involved in the complex, consistent with our previous observation of shifts in NF-κB DNA-binding with these subunits (see Figure 1C). In the reciprocal experiment, only the addition of an anti-STAT3 Ab (Figure 5B, lane 2) or a P-STAT3 (Tyr 705) Ab (lane 3) affected DNA-binding of NF-κB. These super-shift results indicate that NF-κB and P-STAT3 are physically associated. For further verification, we performed Co-IP and Western blotting for P-STAT3 or the p50 subunit of NF-κB. In keeping with the super-shift results, NF-κB and P-STAT3 were co-immunoprecipitated (Figure 5C). Thus, NF-κB and STAT3 reside in the same complex in iMycEμ-1 cells.
NF-κB and STAT3 associate with one another physically in iMycEμ-1 cells. (A and B) EMSA super-shift assays performed with STAT3-specific probes and NF-κB-specific Abs (A), or NF-κB-specific probes and STAT3-specific Abs (B). Abs were specific for NF-κB subunits, Tyr-705 phosphorylated STAT3 (P-STAT3) and total STAT3, as indicated. Abs against SP1 and Myc were used as negative controls. (C) Co-IP and Western blot showing co-immunoprecipitation of NF-κB p50 and P-STAT3. Abs used for immunoprecipitations (IP) and Western blotting (WB) are designated. Images are representative, and image splicing was only carried out only for the same experiment, the same gel and the same exposure times.
AKT is aberrantly activated in LBLs and iMycEμ-1 cells
Having observed signaling crosstalk between constitutively activated NF-κB and STAT3, we investigated the role of some other major signaling pathways in LBLs and iMycEμ-1 cells. Given that PI3K, mTOR and MAPK signaling are important for cell survival and proliferation [21–25], we examined activation of these pathways. The PI3K downstream effector AKT was phosphorylated on both Ser-473 and threonine (Thr)-308 in nearly all LBLs and iMycEμ-1 cells, indicating that it was constitutively activated (Figure 6A). In contrast, phosphorylated forms of ERK, p38 and p70S6K were not readily apparent, indicating that the MAPK and mTOR signaling pathways were not activated. In many types of tumors, the loss or mutation of PTEN leads to elevated activity of the PI3K/AKT pathway [47]. Thus, we evaluated the PTEN levels in LBLs and iMycEμ-1 cells by Western blotting and RT-PCR. PTEN protein or mRNA remained unchanged compared to levels in normal splenic B cells (Figure 6B and 6C, respectively). Activation of AKT from these particular tumor samples and quantitation of PTEN mRNA are shown in additional file 3. Sequencing of PTEN showed no mutation in the Pten gene in either LBLs or iMycEμ-1 cells (data not shown). Additionally, because activating mutations of PIK3CA can result in the constitutive phosphorylation and activation of AKT [48], we sequenced the Pik3ca gene. However, we did not find mutations in this gene in any LBLs or iMycEμ-1 cells (data not shown). These results suggest that constitutive activation of the AKT, but not mTOR or MAPK, pathways is involved in the pathogenesis of iMycEμ lymphoma, independent of loss or mutation of either Pten or Pik3ca.
AKT is constitutively phosphorylated, in a PTEN independent-manner, in a majority of LBLs and iMycEμ-1 cells. (A) Western blot analysis of the activating-phosphorylation status of key proteins of the PI3K (AKT, P-AKT S473 and T308), MAPK (ERK 1/2, P-ERK1/2, total p 38, P-p 38) and mTOR (p70S6K, P-p70S6K) signaling pathways. Positive controls for P-ERK1/2, P-p38 and P-p70S6K were from extracts of UV-treated HeLa cells, NIH 3T3 cells and insulin-treated MCF-7 cells, respectively. (B and C) Levels of PTEN protein (B) and mRNA (C) in LBLs and iMycEμ-1. α-tubulin and β-actin served as loading controls, respectively. "C" denotes control BL6 splenic B cells.
PI3K/AKT is important for the proliferation and survival of iMycEμ-1 cells and is linked to the NF-κB and STAT3 activation, as well as to Myc regulation
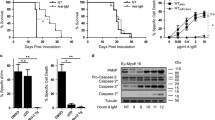
To determine whether constitutive activation of the PI3K/AKT pathway plays a critical role in the proliferation and survival of iMycEμ-1 cells, we cultured them in the presence of the PI3K inhibitor LY294002 (LY). Treatment with LY substantially reduced phosphorylation of AKT (Figure 7A), and resulted in growth suppression (Figure 7B, see additional file 4A) and apoptosis (Figure 7C, see additional file 4B). In keeping with the Western blot results (see Figure 6A), inhibition of ERK by PD98059 (PD), of p38 by SB203580 (SB), of mTOR by rapamycin (Rap), or of JNK by AEG 3482 (AEG) had a marginal to no effect on iMycEμ-1 cell proliferation (Figure 7B, see additional file 4B and 4C). These results show that the PI3K/AKT pathway, but not the MAPK or mTOR pathways, plays an important role in the proliferation and survival of iMycEμ-1 cells. The requirement of PI3K/AKT signaling for constitutive activation of NF-κB, STAT3 and Myc was then examined by EMSA. Inhibition of PI3K significantly reduced NF-κB, STAT3 and Myc activity (Figure 7D) and also led to a reduction of Myc protein (Figure 7E). These effects were identical to those seen following the inhibition of either NF-κB (see Figure 3) or STAT3 (see Figure 4) alone, strongly suggesting crosstalk amongst PI3K/AKT, NF-κB and STAT3.
PI3K inhibition diminishes NF-κB, STAT3 and Myc activity in iMycEμ-1 cells, and reduces their proliferation and survival. (A) Western blot showing the levels of total AKT, PTEN and phosphorylated AKT (S473 and T308) after treatment with LY294002 (LY). α-tubulin was used as a loading control. (B) MTS/PMS assay after treatment with vehicle control, LY, PD98059 (PD), SB203580 (SB), rapamycin (Rap), or AEG 3482 (AEG) at the indicated concentrations. Data were normalized to DMSO-treatment controls, and error bars represent the standard deviation from a representative experiment performed in triplicate. (C) Representative FACS analyses on LY- (open grey histogram) or DMSO- (filled black histogram) treated cells showing an increase in sub-G0/G1 DNA, as assessed by propidium idodide (PI) staining (left panel), and apoptosis as assessed by increases in both Annexin V (middle panel) and activated caspase 3 (right panel) staining. (D) EMSA showing reduced DNA-binding activity of NF-κB, STAT3 and Myc after treatment with LY, but not PD, SB, AEG or Rap. (E) Western blot demonstrating reduced Myc protein levles after inhibition of PI3K; α-tubulin served as a loading control. (F) NF-κB, STAT3 and Myc DNA-binding activity is reduced in a time-dependent manner after PI3K is inhibited with LY. The incubation time with small-molecule inhibitors was 24 hours unless otherwise indicated.
So far our studies had only looked at a snapshot of transcription-factor activity, so we evaluated whether the activity of NF-κB, STAT3 and/or Myc were temporally regulated as a result of PI3K signaling in iMycEμ-1 cells. Differential timing could hint at the order in which these transcription factors might influence one another. The DNA-binding activity of NF-κB and STAT3 diminished with identical kinetics, beginning about six hours after treatment with LY (Figure 7F). Notably, the inhibition of Myc activity was delayed by about two hours compared to inhibition by NF-κB and STAT3 (Figure 7F). These results are in harmony with the possibility that signaling progresses from PI3K to NF-κB and STAT3, which then regulate Myc.
PI3K, NF-κB and/or STAT3 inhibitors have an additive, rather than synergistic, inhibitory effect on iMycEμ-1 cell proliferation
Co-treatment with inhibitors of different signaling pathways can provide useful information regarding intracellular pathway linkage and signal transduction. Because our results have shown that inhibition of any one pathway - PI3K, NF-κB or STAT3 - suppresses proliferation and causes apoptosis, we tested whether co-treatment with inhibitors against these pathways leads to synergitic effects, as has been reported for to be the case for NF-κB and STAT3 [49]. Synergism between these inhibitors would indicate that the target genes (not evaluated here) elicited by NF-κB and STAT3 individually have a greater effect on cell survival and proliferation than the set of target genes elicited by convergent NF-κB/STAT3 signaling. To test this possibility, we cultured iMycEμ-1 cells with low doses of LC, WHI or LY, which individually cause only a very weak or a modest inhibition of proliferation (Figure 8, lanes 2-4). Regardless of the co-treatment combination, an additive, rather than synergistic, effect was observed (Figure 8, compare lane 5 to lanes 2 and 3, lane 6 to lanes 2 and 4, lane 7 to lanes 3 and 4). Considering that there is a certain dependence of both NF-κB and STAT3 on PI3K signaling (see Figure 7), and that NF-κB and STAT3 are physically located in the same molecular complex (see Figure 5), these results suggest that PI3K, NF-κB and STAT3 converge in Myc-driven lymphoma.
Co-treatment with small-molecule inhibitors of NF-κB, STAT3 and/or PI3K additively inhibits the proliferation of iMycEμ-1 cells. MTS/PMS cell proliferation assay after 24-hour treatment with low doses of LY, LC and WHI in isolation or in various combinations, as indicated. Box at the bottom gives the average percent (%) growth inhibition. Data were normalized to DMSO-treatment controls, and error bars represent the standard deviation from a representative experiment performed in triplicate.
Discussion
An enhanced understanding of the signal transduction pathways underlying the development of B-cell neoplasms is an important step towards identifying novel targets for tumor therapy and prevention. Although previous studies have demonstrated that NF-κB, STAT3 and/or PI3K play critical roles in growth control, survival, and chemotherapy resistance of B-cell and plasma-cell neoplasms [50–52], the precise function of NF-κB, STAT3 and/or PI3K in the development of these tumors is not completely understood. In this study, we used the iMycEμ LBL model to uncover signaling crosstalk between NF-κB, STAT3 and PI3K signaling. To our knowledge, this is the first report of crosstalk amongst these pathways in B lymphoma cells. We found that constitutive activation of the PI3K/AKT, but not the mTOR or MAPK pathways, was found to be at least partially responsible for aberrant NF-κB and STAT3 activity. Inhibition of NF-κB, STAT3 or PI3K signaling in iMycEμ B cells, respectively, led to growth suppression, apoptosis and downregulation of Myc. Combined inhibition had an additive effect on proliferation, suggesting that NF-κB and STAT3 converge downstream of PI3K. Our finding that NF-κB and STAT3 are physically associated in iMycEμ-1 B cells supports this interpretation. Signaling crosstalk of NF-κB, STAT3 and PI3K may play an important role in Myc-induced B-cell lymphoma in mice.
The finding that NF-κB, STAT3 and PI3K are constitutively activated in LBLs and iMycEμ-1 cells is in keeping with the aberrant activity of these pathways observed in various types of B cell neoplasms. Constitutive activation of NF-κB has frequently been observed in follicular lymphoma [53, 54], DLBCL [55], mucosa-associated lymphoid tissue (MALT) lymphoma [56], multiple myeloma (MM) [57], and mantle-cell lymphoma (MCL), as well as MCL cell lines, in which inhibition of this constitutive activation induces growth arrest and apoptosis [58–60]. Aberrant STAT3 activation has been documented in MM [61], Hodgkin's disease [62], anaplastic lymphoma kinase-positive (ALK) DLBCL [63], and activated B-cell (ABC) DLBCL, in which JAK2/STAT3 inhibitors trigger arrest and apoptosis [40]. Activation of the PI3K pathway is one of the most common defects in human malignancies, including Burkitt's lymphoma, MCL, and Hodgkin's lymphoma [21–25]. The repeated discovery of the involvement of NF-κB, STAT3 and PI3K in distinct forms of B-cell neoplasias underscores the importance of these signaling pathways in B-cell transformation.
Several findings support crosstalk among NF-κB, STAT3 and PI3K signaling in the iMycEμ system. Inhibition of NF-κB abrogated constitutive STAT3 activity, inhibition of STAT3 reciprocally reduced constitutive NF-κB activity, and inhibition of PI3K suppressed activation of both NF-κB and STAT3 in iMycEμ-1 cells. When inhibitor combinations affecting NF-κB and STAT3 or either and PI3K were applied, additive suppression of proliferation was observed, indicating that the NF-κB and STAT3 pathways converge. The physical association between the active forms of NF-κB and STAT3 in iMycEμ-1 cells provides direct evidence for such crosstalk and convergence. Partial characterization of this complex revealed interactions between the NF-κB subunits p50, p65, and/or c-Rel, either directly or indirectly, with phosphorylated STAT3. The exact compositions of the complexes, and the ultimate functions of these interactions, are not yet defined. Although crosstalk among transcription factors is a common mode of gene regulation, and several studies have already reported physical and functional interactions between NF-κB and STAT3 in various cell types [42–46, 64–66], to our knowledge, this is the first description of a physical association between NF-κB and STAT3 in neoplastic B cells. A recent study showed that constitutive STAT3 activity can maintain constitutive NF-κB activity in solid tumors [46], and our finding supports the possibility of a reciprocal activity of NF-κB and STAT3 in the maintenance of hematopoietic tumors.
We have explored the potential involvement of other pathways in the proliferation and survival of iMycEμ -1 cells and on NF-κB and STAT3 signaling, but found that only the PI3K pathway was involved. It is very interesting that the Eμ-myc model of B-cell lymphoma, one of the earliest transgenic mice ever developed to still be widely used today [67], also showed a requirement for PI3K, but not mTOR or ERK, activity in mitogen-induced B-cell growth [68]. This suggests that the PI3K pathway may be a key modulator of Myc-driven B cell lymphomagenesis. Moreover, inhibition of PI3K abrogated STAT3 and NF-κB activity, and simultaneous inhibition of PI3K with NF-κB or STAT3 resulted in an additive growth inhibition, implying that PI3K functions upstream of NF-κB and STAT3 in iMycEμ B cells. To follow up on how PI3K might be constitutively activated, we assessed the known causes of aberrant PI3K activity - loss or mutation of Pten[69–71] or mutation of Pi3kca[47, 48, 72, 73] - but did not find these alterations in either LBLs or iMycEμ-1 cells. This finding is consistent with other studies indicating that neither PTEN nor PI3KCA is involved in B-cell malignancies [74, 75]. The reason for constitutive activation of PI3K remains to be determined.
In keeping with our results, crosstalk among NF-κB, STAT3 and PI3K signaling is supported in the literature. Notable examples include AKT-mediated phosphorylation of IKK to activate NF-κB [76, 77], IL-2-mediated induction of PI3K upstream of STAT3 activation in primary human T cells [78], and the physical interaction between the PI3K p85 subunit and STAT3 during STAT3 activation [79]. Furthermore, AKT, NF-κB and STAT3 signaling are required for the growth of lymphomas driven by the expression of Epstein-Barr Virus latent membrane protein 1 (EBV-LMP1) [50], and also for the survival of chronic lymphocytic leukemia (CLL) B cells [51]. Intriguingly, several recent reports describe a role for p300, an acetyltransferase, as a potential mediator of signaling crosstalk of NF-κB, STAT3 and PI3K/AKT. AKT-mediated phosphorylation of p300 dramatically increases its acetyltransferase activity and can increase acetylation and full transcriptional activation of p65 [80, 81]. For STAT3, leukemia inhibitory factor (LIF)- or IL6-mediated activation of AKT can lead to phosphorylation of p300, and to subsequent acetylation and activation of STAT3 in 293T and Hep3B cells [65, 82, 83]. Also, acetylation of p65 by p300 is facilitated by STAT3 and can lead to enhanced nuclear localization of p65 [46]. Although proof the involvement of p300 in iMycEμ B-cell neoplasia has not yet been demonstrated, p300 is a prime candidate to link the crosstalk of PI3K, NF-κB, and STAT3 signaling, and is of considerable interest for future studies.
To demonstrate that our results are not unique to iMycEμ-1 cells, we investigated whether similar signal transduction pathways were important for tumor maintenance in other mouse B-lymphoma lines (see additional files 5, 6, & 7). Strikingly similar inhibitor sensitivity was seen in WEHI 231 and iMycEμ-1 cells. In fact, the sort of PI3K/NF-κB/STAT3 signaling crosstalk seen in iMycEμ-1 cells was also observed in WEHI 231 cells when we repeated many of the same experiments (see additional files 6 and 7). These findings argue that our results are not a peculiarity of iMycEμ-1 cells, and also make a strong case for the specificity of the small-molecule inhibitors used in our studies.
Premalignant B cells are difficult to obtain from humans, but mouse models, such as iMycEμ are a ready source of these cells and can be used to elucidate the temporal regulation of molecular events in the course of lymphoma development. We found that NF-κB and STAT3 were already constitutively activated in splenic B cells of iMycEμ mice months before overt tumors developed. The literature would suggest that this early activation of NF-kB and STAT3 is caused by an increase in IL6 and/or IL10 [4, 44, 49]. Our data are novel because they exclude the possibility of elevated IL6 or IL10 from either autocrine or paracrine sources in a pre-tumorigenic state. The reason for constitutive NF-kB and STAT3 activation remains unknown. Intriguingly, NF-κB and STAT3 are known to target Myc [34, 38–41], but Myc protein was only slightly elevated during the premalignant stage in iMycEμ mice. Some of our other results, however, are consistent with Myc as a target downstream of PI3K/NF-κB/STAT3 in tumors of the iMycEμ system. Myc protein was highly elevated during malignancy, and inhibition of any one of the tested effectors of Myc transcription (PI3K, NF-κB or STAT3) resulted in a reduction of Myc protein. Moreover, a loss of Myc activity trailed the reduction of NF-κB and STAT3 activity after PI3K was inhibited in iMycEμ-1 cells. If Myc is upregulated by NF-κB and STAT3, perhaps this occurs at some point between the premalignant and malignant state in iMycEμ B cells. Elucidating the nature of this apparent tumor progression event is ongoing in our laboratory, and will be the subject of a future manuscript.
Conclusions
In summary, we provide evidence that PI3K, NF-κB and STAT3 are interconnected in iMycEμ B cell lymphoma. Constitutive NF-κB and STAT3 activity are dependent upon one another, and both also depend on heightened PI3K activity. Signaling through each of these three molecules is required for tumor maintenance and Myc expression, and combined inhibition results in additive suppression of tumor growth. These findings, together with the fact that NF-κB and STAT3 physically associate with one another in the same complex, support the assertion that NF-κB and STAT3 converge downstream of PI3K in the development of iMycEμ B-cell lymphoma. Our results underscrore the importance of further examination of crosstalk between NF-κB, STAT3 and PI3K in the development of Myc-driven B-cell neoplasia.
References
Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, Dörken B: Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997, 100: 2961-2969. 10.1172/JCI119849
Davis RE, Brown KD, Siebenlist U, Staudt LM: Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001, 194: 1861-1874. 10.1084/jem.194.12.1861
Bassères DS, Baldwin AS: Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene. 2006, 25: 6817-6830. 10.1038/sj.onc.1209942
Squarize CH, M Castilho RM, Sriuranpong V, Pinto DS, Gutkind JS: Molecular Crosstalk between the NF-κB and STAT3 Signaling Pathways in Head and Neck Squamous Cell Carcinoma. Neoplasia. 2006, 8: 733-746. 10.1593/neo.06274
Espinosa I, Briones J, Bordes R, Brunet S, Martino R, Sureda A, Sierra J, Prat J: Activation of the NF-kappaB signalling pathway in diffuse large B-cell lymphoma: clinical implications. Histopathology. 2008, 53: 441-449. 10.1111/j.1365-2559.2008.03139.x
Li Q, Verma IM: NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002, 2: 725-734. 10.1038/nri910
Baeuerle PA, Baltimore D: NF-κB. ten years after. Cell. 1996, 87: 13-20. 10.1016/S0092-8674(00)81318-5
Karin M, Ben-Neriah Y: Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000, 18: 621-663. 10.1146/annurev.immunol.18.1.621
Karin M, Greten FR: NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005, 5: 749-759. 10.1038/nri1703
Siebenlist U, Brown K, Claudio E: Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005, 5: 435-445. 10.1038/nri1629
Calò V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A: STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003, 197: 157-168. 10.1002/jcp.10364
Bowman T, Garcia R, Turkson J, Jove R: STATs in oncogenesis. Oncogene. 2000, 19: 2474-2488. 10.1038/sj.onc.1203527
Joo A, Aburatani H, Morii E, Iba H, Yoshimura A: STAT3 and MITF cooperatively induce cellular transformation through upregulation of c-fos expression. Oncogene. 2004, 23: 726-734. 10.1038/sj.onc.1207174
Akira S, Nishi Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T: Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994, 77: 63-71. 10.1016/0092-8674(94)90235-6
Levy DE, Lee CK: What does Stat3 do?. J Clin Invest. 2002, 109: 1143-1148.
Hirano T, Ishihara K, Hibi M: Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000, 19: 2548-2556. 10.1038/sj.onc.1203551
O'Shea JJ: Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet?. Immunity. 1997, 7: 1-11. 10.1016/S1074-7613(00)80505-1
Decker T, Kovarik P: Serine phosphorylation of STATs. Oncogene. 2000, 19: 2628-2637. 10.1038/sj.onc.1203481
Wen Z, Zhong Z, Darnell JE: Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995, 82: 241-250. 10.1016/0092-8674(95)90311-9
Schuringa JJ, Schepers H, Vellenga E, Kruijer W: Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL6 stimulation. FEBS Lett. 2001, 495: 71-76. 10.1016/S0014-5793(01)02354-7
Cantley LC: The phosphoinositide 3-kinase pathway. Science. 2002, 296: 1655-1657. 10.1126/science.296.5573.1655
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA: Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003, 17: 590-603. 10.1038/sj.leu.2402824
Dutton A, Reynolds GM, Dawson CW, Young LS, Murray PG: Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005, 205: 498-506. 10.1002/path.1725
Fillmore GC, Wang Q, Carey MJ, Kim CH, Elenitoba-Johnson KS, Lim MS: Expression of Akt (protein kinase B) and its isoforms in malignant lymphomas. Leuk Lymphoma. 2005, 46: 1765-1773. 10.1080/10428190500159944
Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, Quintanilla-Martinez L, Raffeld M: Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006, 108: 1668-1676. 10.1182/blood-2006-04-015586
Li L, Ittmann MM, Ayala G, Tsai MJ, Amato RJ, Wheeler TM, Miles BJ, Kadmon D, Thompson TC: The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005, 8: 108-18. 10.1038/sj.pcan.4500776
Di Cristofano A, Pandolfi PP: The multiple roles of PTEN in tumor suppression. Cell. 2000, 100: 387-390. 10.1016/S0092-8674(00)80674-1
Giri D, Ittmann M: Inactivation of the PTEN tumor suppressor gene is associated with increased angiogenesis in clinically localized prostate carcinoma. Hum Pathol. 1999, 30: 419-424. 10.1016/S0046-8177(99)90117-X
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW: Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998, 95: 29-39. 10.1016/S0092-8674(00)81780-8
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL: Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000, 60: 1541-1545.
Park SS, Kim JS, Tessarollo L, Owens JD, Peng L, Han SS, Chung ST, Torrey TA, Cheung WC, Polakiewicz RD, McNeil N, Ried T, Mushinski JF, Morse HC, Janz S: Insertion of c-Myc into Igh induces B-cell and plasma-cell neoplasms in mice. Cancer Res. 2005, 65: 1306-1315. 10.1158/0008-5472.CAN-04-0268
Han SS, Shaffer AL, Peng L, Chung ST, Lim JH, Maeng S, Kim JS, McNeil N, Ried T, Staudt LM, Janz SL: Molecular and cytological features of the mouse B-cell lymphoma line iMycEmu-1. Mol Cancer. 2005, 9: 4-40.
Lee H, Arsura M, Wu M, Duyao M, Buckler AJ, Sonenshein GE: Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. J Exp Med. 1995, 181: 1169-1177. 10.1084/jem.181.3.1169
Wu M, Arsura M, Bellas RE, FitzGerald MJ, Lee H, Schauer SL, Sherr DH, Sonenshein GE: Inhibition of c-myc expression induces apoptosis of WEHI 231 murine B cells. Mol Cell Biol. 1995, 16: 5015-5025.
Wu M, Lee H, Bellas RE, Schauer SL, Arsura M, Katz D, FitzGerald MJ, Rothstein TL, Sherr DH, Sonenshein GE: Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 1996, 15: 4682-4690.
Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S: Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 1999, 93: 152-161. 10.1006/clim.1999.4769
Han SS, Chung ST, Robertson DA, Chelvarajan RL, Bondada S: CpG oligodeoxynucleotides rescue BKS-2 immature B cell lymphoma from anti-IgM-mediated growth inhibition by up-regulation of egr-1. Int Immunol. 1999, 11: 871-879. 10.1093/intimm/11.6.871
Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI: Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008, 14: 447-57. 10.1016/j.ccr.2008.10.018
Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T: STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999, 189: 63-73. 10.1084/jem.189.1.63
Ding BB, Yu JJ, Yu RY, Mendez LM, Shaknovich R, Zhang Y, Cattoretti G, Ye BH: Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008, 111: 1515-1523. 10.1182/blood-2007-04-087734
Duyao MP, Buckler AJ, Sonenshein GE: Interaction of an NF-kB-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci USA. 1990, 87: 4727-4731. 10.1073/pnas.87.12.4727
Yu Z, Zhang W, Kone BC: Signal transducers and activators of transcription 3 STAT3 inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor κB. Biochem J. 2002, 367: 97-105. 10.1042/BJ20020588
Yu Z, Kone BC: The STAT3 DNA-binding domain mediates interaction with NF-kappaB p65 and iuducible nitric oxide synthase transrepression in mesangial cells. J Am Soc Nephrol. 2004, 15: 585-591. 10.1097/01.ASN.0000114556.19556.F9
Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR: Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007, 21: 1396-1408. 10.1101/gad.1553707
Yoshida Y, Kumar A, Koyama Y, Peng H, Arman A, Boch JA, Auron PE: Interleukin 1 activates STAT3/nuclear factor-κB crosstalk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004, 279: 1768-1776. 10.1074/jbc.M311498200
Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H: Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009, 15: 283-293. 10.1016/j.ccr.2009.02.015
Bellacosa A, Kumar CC, Di Cristofano A, Testa JR: Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005, 94: 29-86. 10.1016/S0065-230X(05)94002-5
Samuels Y, Diaz LA, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE: Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005, 7: 561-573. 10.1016/j.ccr.2005.05.014
Lam LT, Wright G, Davis RE, Lenz G, Farinha P, Dang L, Chan JW, Rosenwald A, Gascoyne RD, Staudt LM: Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008, 111: 3701-3713. 10.1182/blood-2007-09-111948
Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB: Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007, 109: 2293-2302. 10.1182/blood-2006-02-003988
Shair KH, Bendt KM, Edwards RH, Bedford EC, Nielsen JN, Raab-Traub N: EBV latent membrane protein 1 activates Akt, NFkappaB, and Stat3 in B cell lymphomas. PLoS Pathog. 2007, 3: e166- 10.1371/journal.ppat.0030166
Ghosh AK, Kay NE, Secreto CR, Shanafelt TD: Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009, 15: 1250-1258. 10.1158/1078-0432.CCR-08-1511
Heckman CA, Mehew JW, Boxer LM: NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene. 2002, 21: 3898-3908. 10.1038/sj.onc.1205483
Heckman CA, Cao T, Somsouk L, Duan H, Mehew JW, Zhang CY, Boxter LM: Critical elements of the immunoglobulin heavy chain gene enhancers for deregulated expression of bcl-2. Cancer Res. 2003, 63: 6666-6673.
Shaffer AL, Rosenwald A, Staudt LM: Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002, 2: 920-932. 10.1038/nri953
Zhou H, Du MQ, Dixit VM: Constitutive NF-kappaB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005, 7: 425-431. 10.1016/j.ccr.2005.04.012
Ni H, Ergin M, Huang Q, Qin JZ, Amin HM, Martinez RL, Saeed S, Barton K, Alkan S: Analysis of expression of nuclear factor kappa B (NF-kappa B) in multiple myeloma: downregulation of NF-kappa B induces apoptosis. Br J Haematol. 2001, 115: 279-286. 10.1046/j.1365-2141.2001.03102.x
Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ: Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003, 171: 88-95.
Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ: Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood. 2006, 107: 4540-4548. 10.1182/blood-2005-10-4042
Keutgens A, Robert I, Viatour P, Chariot A: Deregulated NF-kappaB activity in haematological malignancies. Biochem Pharmacol. 2006, 72: 1069-1080. 10.1016/j.bcp.2006.06.011
Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC: Focus on multiple myeloma. Cancer Cell. 2004, 6: 439-444. 10.1016/j.ccr.2004.10.020
Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I, Heinrich PC, Diehl V, Tesch H: STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001, 98: 762-770. 10.1182/blood.V98.3.762
Momose S, Tamaru J, Kishi H, Mikata I, Mori M, Toyozumi Y, Itoyama S: Hyperactivated STAT3 in ALK-positive diffuse large B-cell lymphoma with clathrin-ALK fusion. Hum Pathol. 2009, 40: 75-82. 10.1016/j.humpath.2008.06.009
Agrawal A, Cha-Molstad H, Samols D, Kushner I: Overexpressed nuclear factor-kappaB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3. Immunology. 2003, 108: 539-547. 10.1046/j.1365-2567.2003.01608.x
Yuan ZL, Guan YJ, Chatterjee D, Chin YE: Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005, 307: 269-273. 10.1126/science.1105166
Hagihara K, Nishikawa T, Sugamata Y, Song J, Isobe T, Taga T, Yoshizaki K: Essential role of STAT3 in cytokine-driven NF-κB-mediated serum amyloid A gene expression. Genes Cells. 2005, 10. 1051-1063. 10.1111/j.1365-2443.2005.00900.x
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL: The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985, 318: 533-538. 10.1038/318533a0
Grumont RJ, Strasser A, Gerondakis S: B cell growth is controlled by phosphatidylinosotol 3-kinase-dependent induction of Rel/NF-kappaB regulated c-myc transcription. Mol Cell. 2002, 10: 1283-1294. 10.1016/S1097-2765(02)00779-7
Lee S, Choi EJ, Jin C, Kim DH: Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005, 97: 26-34. 10.1016/j.ygyno.2004.11.051
Sakai A, Thieblemont C, Wellmann A, Jaffe ES, Raffeld MP: PTEN gene alterations in lymphoid neoplasms. Blood. 1998, 92: 3410-3415.
Samuels Y, Velculescu VE: Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004, 3: 1221-1224.
Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM, Trink B, Ladenson PW, Sidransky D, Xing M: Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 2005, 90: 4688-4693. 10.1210/jc.2004-2281
Bertelsen BI, Steine SJ, Sandvei R, Molven A, Laerum OD: Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006, 118: 1877-1883. 10.1002/ijc.21461
García JF, Camacho FI, Morente M, Fraga M, Montalbán C, Alvaro T, Bellas C, Castaño A, Díez A, Flores T, Martin C, Martinez MA, Mazorra F, Menárguez J, Mestre MJ, Mollejo M, Sáez AI, Sánchez L, Piris MA: Spanish Hodgkin Lymphoma Study Group. Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood. 2003, 101: 681-689. 10.1182/blood-2002-04-1128
Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S: Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008, 17: 159-65. 10.1097/PDM.0b013e31815d0588
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB: NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999, 401: 82-85. 10.1038/43466
Romashkova JA, Makarov SS: NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999, 401: 86-90. 10.1038/43474
Fung MM, Rohwer F, McGuire KL: IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells. Cell Signal. 2003, 15: 625-636. 10.1016/S0898-6568(03)00003-2
Krasil'nikov M, Shatskaya V: Signal transducer and activator of transcription-3 and phosphatidylinositol-3 kinase as coordinate regulators of melanoma cell response to glucocorticoid hormones. J Steroid Biochem Mol Biol. 2002, 82: 369-376. 10.1016/S0960-0760(02)00223-6
Chen LF, Fischle W, Verdin E, Greene WC: Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001, 293: 1653-1657. 10.1126/science.1062374
Huang WC, Chen CC: Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol. 2005, 25: 6592-6602. 10.1128/MCB.25.15.6592-6602.2005
Ohbayashi N, Ikeda O, Taira N, Yamamoto Y, Muromoto R, Sekine Y, Sugiyama K, Honjoh T, Matsuda T: LIF- and IL-6-induced acetylation of STAT3 at Lys-685 through PI3K/Akt activation. Biol Pharm Bull. 2007, 30: 1860-4. 10.1248/bpb.30.1860
Ray S, Boldogh I, Brasier AR: STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology. 2005, 129: 1616-1632. 10.1053/j.gastro.2005.07.055
Acknowledgements
This work was supported in part by an Aiming For A Cure Foundation Grant from the Holden Comprehensive Cancer Center and Award No. P50CA097274 from the NCI. Dong-Ju Son in this work was supported by the Korea Research Foundation Grant (KRF-2007-357-E00032) funded by the Korean Government (MOEHRD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SSH designed the study, performed most experiments, and wrote the article. DJS conducted proliferation assays, Co-IPs and the cytokine array experiments. HY performed Western blot analysis. VST contributed critical insights and edited the article. LP conducted FACS analysis. STC cultured cells and conducted proliferation assays. JK performed Western blot analysis for c-Myc. ESP contributed critical insights. SJ designed the study, evaluated results, and wrote and approved the article. All authors have read and approved the final manuscript.
Electronic supplementary material
12943_2009_612_MOESM1_ESM.JPEG
Additional file 1:NF-κB and STAT3 are constitutively activated in splenic B cells of young iMycEμ mice. (A and B) EMSA showing an increase in DNA-binding for NF-κB (A) and STAT3 (B), respectively, beginning at one month of age and continuing through four months of age. Nuclear extracts from BL6-derived splenic B cells were used as a control (C). (JPEG 31 KB)
12943_2009_612_MOESM2_ESM.JPEG
Additional file 2:The Myc probe is specific. (A) EMSA competition assay showing that 30-fold excess unlabeled probe successfully competes for Myc DNA-binding but a mutated probe does not. (B) Super-shift assay showing a loss of Myc DNA-binding that is specific for the Myc Ab but not for STAT3, p 50 or p 65 Abs. (JPEG 43 KB)
12943_2009_612_MOESM3_ESM.JPEG
Additional file 3:AKT is activated but PTEN message does not change in LBLs or iMycEμ-1 cells. (A) Western blotting reveals the levels of total AKT protein and of phosphorylated AKT (S473 and T308) in the same samples, as in Figure 6B of Han et al. (2009). (B) Quantitation of PTEN mRNA, as shown in Figure 6C of Han et al. (2009). (JPEG 43 KB)
12943_2009_612_MOESM4_ESM.JPEG
Additional file 4:Inhibition of the PI3K pathway induces growth arrest and apoptosis of iMycEμ-1 cells. (A) MTS/PMS anlysis reveals a dose-dependent decrease in cell proliferation after cells are treated with LY. Data were normalized to DMSO-treatment controls, and error bars represent the standard deviation from a representative experiment performed in triplicate. (B) DNA fragmentation was observed after treatment with LY, but not Rap, PD or SB, respectively. (C) Flow cytometry-based analyses of DNA content (PI; top row), as well as Annexin V (middle row) and cleaved caspase 3 (bottom row) levels, showing that treatment with PD, SB or Rap (open gray histogram) did not result in significant differences from untreated controls (filled black histogram). Treatments were for 24 hours and the inhibitor concentrations are indicated. (JPEG 100 KB)
12943_2009_612_MOESM5_ESM.JPEG
Additional file 5:Wehi 231cells are very similar to iMycEμ-1 cells with regard to NF-κB, STAT3 and PI3K signaling. (A) EMSA showing constitutive activation of NF-κB, STAT3 and Myc in mouse BCL cell lines, as indicated. "C" denotes control BL6 splenic B cells. (B) Western blot comparing protein levels of AKT, P-AKT (S473 and T308), PTEN and α-tubulin in mouse BCL lines, as indicated. (C) MTS/PMS assay for proliferation of designated mouse BCL lines after treatment with vehicle control, LY, PD, SB, AEG, Rap, LC or WHI for 24 hours at the given concentrations. Data were normalized to DMSO controls, and error bars represent the standard deviation from a representative experiment performed in triplicate. (JPEG 114 KB)
12943_2009_612_MOESM6_ESM.JPEG
Additional file 6:In Wehi 231 cells, crosstalk among NF-κB, STAT3 and PI3K appears to regulate Myc. (A) EMSA revealing that binding of NF-κB, STAT3 and Myc to DNA is sensitive to inhibition of PI3K (LY), NF-κB (LC), STAT3 (WHI) and JNK (AEG), but not PD, SB or Rap. (B) NF-κB, STAT3 and Myc DNA-binding activity is reduced in a time-dependent manner after PI3K is inhibited with LY. (C and D) EMSA super-shift assays performed with STAT3-specific probes and NF-κB-specific Abs (C) or NF-κB-specific probes and STAT3-specific Abs (D), respectively. Abs were specific for subunits of NF-κB, Tyr-705 phosphorylated STAT3 (P-STAT3) and total STAT3 as indicated. SP1 and Myc Abs were used as negative controls. (E) Co-IP and Western blot showing co-immunoprecipitation of NF-κB p50 and P-STAT3. Abs used for immunoprecipitations (IP) and Western blotting (WB) are designated. The incubation time with small-molecule inhibitors was 24 hours unless otherwise noted. (JPEG 120 KB)
12943_2009_612_MOESM7_ESM.JPEG
Additional file 7:Co-treatment with small-molecule inhibitors of NF-κB, STAT3 and/or PI3K additively inhibits proliferation of Wehi 231 cells. MTS/PMS cell proliferation assay after cell treatment with low doses of LY, LC and WHI, either in isolation or in various combinations, for 24 hours. Box at bottom gives the average percent (%) growth inhibition. Data were normalized to DMSO controls, and error bars represent the standard deviation from a representative experiment performed in triplicate. (JPEG 43 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Han, SS., Yun, H., Son, DJ. et al. NF-κB/STAT3/PI3K signaling crosstalk in iMycEμ B lymphoma. Mol Cancer 9, 97 (2010). https://doi.org/10.1186/1476-4598-9-97
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-4598-9-97