Abstract
Introduction
Antibiotic resistance decreases success of Helicobacter pylori (Hp) eradication. Recently published results show low rate of resistance and better compliance with moxifloxacin based regiments.
Aims&methods
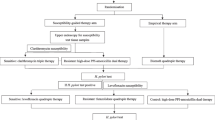
Whether 7 days moxifloxacin with lansoprasole and amoxycillin can be compared with 10 days moxifloxacin with lansoprasole and amoxycillin according to moxifloxacin resistance. Patients with non-ulcer dyspepsia who had culture and histology positive Hp infection (n = 150) were randomly assigned into two groups. The first group (n = 75) received moxifloxacin 400 mg/d during 7 days and the other (n = 75) received moxifloxacin 400 mg/d during 10 days. All patients received amoxycillin 1 g twice daily, lansoprasole 30 mg twice daily. All Hp cultures were tested for sensitivity to moxifloxacin.
Results
138 patients (92%) completed the study, 68 in the first group and 70 in the second. Eradication rates were 84% (57/68) and 76% (57/75) in the 7 days moxifloxacin group and 90% and 84% in the second group (63/70, 63/75) according to the PP and ITT analysis; p = n.s. Among 129 patients (86% of study group), 6% of strains were primary resistant to moxifloxacin.
Eradication of moxifloxacin sensitive/resistant strains was 98%/66%, p < 0.05
Conclusion
According to our results we recommend 7 days moxiflixacin based triple therapy.
Similar content being viewed by others
Introduction
During the last few decades a large number of surveys have been conducted to show the importance of Helicobacter pylori infection as the main etiologic factor in chronic active gastritis, peptic ulcers and gastric malignancies [1]. European guidelines for Helicobacter pylori eradication recommend a triple regimen, combining proton pump inhibitor (PPI), claritromycin, and either amoxicillin or metronidazole as the first-line treatment [2, 3]. Resistance to antibiotics is a major cause of eradication failure [4–6]. Currently available first-line anti H. pylori therapies may fail in up to 30% of patients [7, 8] leading to significant increase of antimicrobial resistance, particularly against key antibiotics, claritromycin and/or metronidazole [9, 10]. Resistance to amoxicillin and tetracycline is generally very low, even absent and is clinically insignificant [11]. To avoid resorting to rescue therapies, recent clinical trials have demonstrated that fluoroquinolone-based triple regimens may provide important options for the treatment [12–14]. Moxifloxacin-based triple regimens have been shown as a promising alternative by proving to have a good efficacy in the treatment of H. pylori infection [14–16]
Moxifloxacin is a second-generation fluoroquinolone widely used to treat respiratory and skin infections [17]. It is rapidly absorbed after oral administration, penetrates tissues well, and its half-life of 9-16 hours allows a single daily dose. The drug is well tolerated with only mild gastrointestinal disturbances (nausea and diarrhoea) as the most common side-effects [18].
According to these findings, in 2007 our group already investigated and proved the efficacy of one week of moxifloxacin-based treatment comparing with the standard treatment therapy [19].
The aim of this study was to determine the impact of moxifloxacine resistance and period of treatment on the effectiveness of a treatment regimen for eradication of H. pylori infection.
Petients, matherials and methods
The study was conducted at the Department of Hepatogastroenterology, Internal Medical Clinic in the Clinical Hospital "Sveti Duh", Zagreb, Croatia between June 2007 and June 2008.
This was an open, randomized, comparative clinical trial with parallel groups of patients.
Before entering the study, each patient reviewed and signed an informed consent form approved by the Local Ethics Committee. Standards of Good Clinical Practice and The declaration of Helsinki were followed.
Patients' recruitment
Consecutive patients of either sex and at least 18 years of age were recruited from those referred to Endoscopy Services for diagnostic upper-gastrointestinal endoscopy for evaluation of dyspeptic symptoms, and found to suffer from non-ulcer dyspepsia and concomitant H. pylori infection.
Patients were not suitable for the study if one of the criteria was met: diagnostically duodenal or gastric ulcers, gastrointestinal bleeding, previous attempts to eradicate H. pylori and usage of antibiotics and/or anti-gastric acid therapy within the previous 30 days and/or bismuth therapy within 90 days and usage of non-steroidal anti-inflammatory drugs, corticosteroids or gold-based drugs. The presence of severe concurrent diseases (cardio respiratory, renal, hepatic, neurological, pulmonary, metabolic, hematological and endocrine and suspected or confirmed malignancy), a condition associated with poor compliance (alcohol and drug abuse) pregnancy and breastfeeding were also exclusion criteria.
Procedures used to diagnose Helicobacter pylori infection
Endoscopy was performed with an OlympusR FB20 endoscope (Olympus Optical Co., Tokyo, Japan) before treatment and 4-6 weeks after the treatment. Patients fasted a night before testing. Eight gastric mucosal biopsies per patient were taken with biopsy forceps (Olympus FB 24-K) in order to perform rapid urease testing (CLO test), histology and culture.
One sample each from antrum and corpus were used for the CLO test (Delta West Ltd. Bently Western Australia) which was considered positive if there was change in color within 24 hours. Histology was performed on two biopsies, each from the antrum and corpus, following the guidelines of the Sydney System. (2004- 12-Price AB 1991)
The biopsies were placed in separate tubes containing buffered neutral 3.7% formaldehyde solution. Hematoxylineosin stain was used to grade gastritis and Giemsa stain to detect H. Pylori.
One antral and one corpus gastric biopsy samples were taken for culture and put in a sterile receptacle containing before transporting to the microbiology laboratory within two hours after sterile pulverization specimens were placed on to fresh Columbia agar with added 7% horse blood and Iso Vitalex (BBL, Bection-Dickinson, Maryland, USA) and incubated for 3-5 days at 35-37°C under microaerophilic conditions. H. Pylori was identified as gram negative, spiral or curved rods, producing urease, catalase and oxidase.
Condition to enroll the patients in to the study was the following: two of the three tests must be positive. The treatment was considered successful if all biopsy based tests did not show the presence of H. Pylori on post-treatment assessment.
If one test suggested persistence of H. pylori a C- urea test was performed.
The 13C-urea breath test was performed after overnight fast and collection of baseline exhaled-breath sample. To delay gastric empting, each patient got a glass of orange juice (200 ml). Then 75 mg of 13C-urea dissolved in 30 ml of water was administered orally. 30 minutes later, the second breath sample was obtained. All breath samples were analyzed in an isotope-ratio mass spectrometer (IRMS, Wagner Analysen Technik, Bremen, Germany). An increase of 13C02 exceeding the baseline value by more than 4‰ indicated the presence of H. pylori.
Determination of H. pylori resistance
All positive cultures were tested for sensitivity to moxifloxacin and amoxicillin using E-test (AB Biodisk, Sweden), which determined minimum inhibitory concentration (MIC). The grown H. pylori colonies were resuspended in 2 li Dulbecco's modified Eagle's cell culture medium after three to five days. From this suspension 100 microliters were flooded on Columbia agar plates containing 7% horse blood. When the surface had dried, E-stripes were placed on the agar. All plates were incubated under microaerophilic conditions at 37°C as described above. They were red after 48 hours.
The MIC was defined as the lowest concentration including complete growth inhibition. The cut-off points for determining a strain as resistant were as follows: 2 μg/ml for moxifloxacin and 2.25 μml for amoxicillin.
Treatment groups
The patients were distributed randomly to one of the following regimes:
-
1.
LAM 7: lansoprazole 30 mg + amoxicillin 1000 mg + moxifloxacin 400 mg for 7 days
-
2.
LAM 10: lansoprazole 30 mg + amoxicillin 1000 mg + moxifloxacin 400 mg for 10 days
A physical examination was carried out and medical history was taken during the pre-study visit. All three drugs were distributed in packs in 14 single doses and given to patients who were instructed to take drugs together two times a day, after breakfast and dinner. Each patient was asked to monitor and document all symptoms during therapy. Therapy compliance was assessed by pill count at the end of the treatment and defined as intake of >80% of the distributed medication.
Between 4 and 6 weeks after the treatment the follow up was performed, during which all changes in clinical symptoms and signs were documented. At the same time the second endoscopy was arranged, before which the investigators were not familiar with the results of E-test. While assessing the biopsy samples, the microbiologist and the pathologist were blinded to the treatment regimes, distributed to patients.
Statistics
Analysis of efficacy of H. pylori eradication was performed on an intention-to-treat (ITT) and per protocol (PP) basis. The ITT analysis included all enrolled patients who received at least one dose of included drugs. The PP analysis included only those patients who complied with the study protocol (>80% of the assigned medication) and underwent a second endoscopy between 4 and 6 weeks after the treatment. All results of the treatment are expressed as percentages with 95% confidence intervals. The rates of H. pylori eradication in different groups were analyzed by Student's t-test. Statistical significance was taken at p < 0.05. χ2 test was used to compare categorical variables, while the analysis of variance was used to compare the demographic data.
Results
Clinical and demographic variables, compliance and loss from follow up
Between June 2007 and June 2008 a total of 150 patients enrolled in the study. The groups did not differ in baseline characteristics, as summered in Table 1.
The mean age was 52 ± 13. According to gender, 48% were male and 52% were female.
All the patients were included in the ITT analysis. 12 patients (8%) did not complete the study for one of the following reasons: loss from follow up (n = 1), refusal to go under a second endoscopy (n = 3), adverse effects leading to discontinuation of treatment (n = 5), concurred disapproved medication (n = 2) and taking less than 80% of the prescribed medicines (n = 1). Those patients were excluded from PP analysis.
Helicobacter pylori eradication
According to ITT and PP analysis with their respective 95% confidence intervals, H. pylori eradication rates did not show a significant difference between LAM 7 and LAM 10 protocols, as is presented in Table 2.
Primary resistance
MIC determination was successful in samples from 92% (138/150).
Death of the H. pylori strain, fungal overgrowth or negative culture prevented MIC determination with the E-test in samples from 8% (12/150 patients). Among 129 patients (86% of the entire study group), 6% of strains were primary resistant to moxifloxacin. Eradication of moxifloxacin sensitive/resistant strains were 98%/66%, p < 0.005. All 138 strains were sensitive to amoxicillin.
Impact of primary resistance on eradication of H. pylori
Results of our study suggest that the impact of H. pylori resistence to moxifloxacin on eradication is statisticaly significant. There was statistic significance between resistant strains and sensitive strains in LAM 7 protocol (p = 0,006), as well as in LAM 10 group (p = 0.0001).
There was no significant difference between sensitive strains in LAM 7 and sensitive strains in LAM 10 protocol.
Longer period of treatment did not provide significantly higher eradication rate, concerning both, sensitive and resistant strains.
Impact of primary resistance on eradication of H. pylori is shown in Table 3.
Adverse effects and compliance
Most adverse effects were mild to moderate in intensity. Two patients in the LAM 7 group discontinued the treatment because of diarrhoea. One patient in the LAM 10 group discontinued the treatment because of epigasrtic discomfort and two because of diarrhoea. In conclusion, side effects were more frequent with the larger period of drug usage, although the difference was not significant.
Detailed overview of adverse effects is shown in a Table 4 below.
Discussion
Until now, many different antimicrobial agents have been investigated, but choosing the appropriate first-line treatment for the H. pylori treatment remains a challenge.
Apart from antibiotic resistance [4–6], another reason for treatment failure lies in the fact that side-effects, longer period of treatment and amount of pills administrated per day have a negative effect on patients' compliance [20].
For those reasons, it was necessary to develop a new therapy scheme, which will result in higher eradication rates and will include fewer types of antibiotics in therapy with minor dosages and shorter period of treatment which provides better patients' compliance, fewer side-effects and lower expenses of treatment.
In the last few years, new treatment schemes based on fluoroquinolones, such as levofloxacin and moxifloxacin have been investigated all over the world.
Up to now, only few studies have evaluated a combination of levofloxacin, amoxicillin and PPI as first- line regimen. Gisbert end al. Reported in 2007 that 10-day levofloxacin- based combination represents an alternative to clarythromycin [21].
Antos and al. suggested levofloxacin tripple therapy as an option with metronidazole and clarithromycin resistant H. pylori strains [22].
However, recent studies from France, Belgium and Germany suggest that resistance of H. pylori to quinolones has already risen up to 17%, most likely due to widespread use of quinolones in other indications [23–25].
According to Italian investigators, primary resistance to levofloxacin rised up to 30.3% [26], while in Japan it amounts 15%. Indonesian scientists proved that flouroquinolones show lower resistance rates, 6.9% for ciprofloxacin, norfloxacin and ofloxacin, 2.8% for sparfloxacin and gatifloxacin, and 1.4% for levofloxacin and moxifloxacin [27].
In Southern Taiwan resistance to ciprofloxacin and levofloxacin in H. pylori collected from 2004 to 2007 increased significantly compared with the level observed during 1998 to 2003 (2.8% to 11.8%) [28].
Guided by these facts, in 2007, our group assumed and proved that moxifloxacin based treatment is effective and safe option comparing to standard triple regimens due to increased prevalence of claritromycin resistance (10.8%) [16] and metronidazole resistance (33%) [29]. Therefore, it was necessary to invent and to verify a new treatment scheme in our area, which will provide acceptable therapy outcome.
In order to improve the treatment, according to all these findings, our study group conducted this survey to determine an impact of moxifloxacine resistance and therapy duration on the effectiveness of a treatment regimen for eradication of H. pylori infection. Therefore, we compared H. pylori eradication rates, adverse effects and patients' compliance.
The eradication rates of 10-days treatment were higher than those of 7-days treatment by both ITT and PP analyses.
Adverse effects and treatment discontinuation due to adverse effects were more frequent with the larger period of drug usage, although, the difference was not significant.
According to our results we recommend 7 days moxiflixacin based triple therapy because we found no statisticaly significant eradication rates comparing LAM 7 and LAM 10 protocols.
Our assumption is that a low prevalence of fluoroquinolone resistance in Croatia may be associated with the decreased availability and the short time usage of fluoroquinolones in our practice.
In addition, it is essential to investigate the local prevalence of H. pylori resistance to antibiotics before introducing moxifloxacin in practice and, in the future, to detect a possible increase of resistance to moxifloxacin after certain period of drug usage.
References
Suerbaum S, Michetti P: Helicobacter pylori infection. N Engl J Med 2002,347(15):1175-86. 10.1056/NEJMra020542
Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G, European Helicobacter Pylori Study Group (EHPSG): Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther 2002,16(2):167-80. 10.1046/j.1365-2036.2002.01169.x
Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ: Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007,56(6):772-81. 10.1136/gut.2006.101634
Graham DY: Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998,115(5):1272-7. 10.1016/S0016-5085(98)70100-3
Mégraud F, Doermann HP: Clinical relevance of resistant strains of Helicobacter pylori: a review of current data. Gut 1998,43(Suppl 1):S61-5.
Ducóns JA, Santolaria S, Guirao R, Ferrero M, Montoro M, Gomollón F: Impact of clarithromycin resistance on the effectiveness of a regimen for Helicobacter pylori: a prospective study of 1-week lansoprazole, amoxycillin and clarithromycin in active peptic ulcer. Aliment Pharmacol Ther 1999,13(6):775-80. 10.1046/j.1365-2036.1999.00549.x
Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M: Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch Intern Med 1999,159(14):1562-6. 10.1001/archinte.159.14.1562
Della Monica P, Lavagna A, Masoero G, Lombardo L, Crocellá L, Pera A: Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther 2002,16(7):1269-75. 10.1046/j.1365-2036.2002.01244.x
Heep M, Kist M, Strobel S, Beck D, Lehn N: Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur J Clin Microbiol Infect Dis 2000,19(7):538-41. 10.1007/s100960000288
Wang WH, Wong BC, Mukhopadhyay AK, Berg DE, Cho CH, Lai KC, Hu WH, Fung FM, Hui WM, Lam SK: High prevalence of Helicobacter pylori infection with dual resistance to metronidazole and clarithromycin in Hong Kong. Aliment Pharmacol Ther 2000,14(7):901-10. 10.1046/j.1365-2036.2000.00795.x
Mégraud F: H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004,53(9):1374-84. 10.1136/gut.2003.022111
Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, Gasbarrini A, Armuzzi A, Zocco MA, Santarelli L, Arancio F, Gasbarrini G: Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther 2000,14(10):1339-43. 10.1046/j.1365-2036.2000.00846.x
Di Caro S, Zocco MA, Cremonini F, Candelli M, Nista EC, Bartolozzi F, Armuzzi A, Cammarota G, Santarelli L, Gasbarrini A: Levofloxacin based regimens for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 2002,14(12):1309-12. 10.1097/00042737-200212000-00004
Di Caro S, Ojetti V, Zocco MA, Cremonini F, Bartolozzi F, Candelli M, Lupascu A, Nista EC, Cammarota G, Gasbarrini A: Mono, dual and triple moxifloxacin-based therapies for Helicobacter pylori eradication. Aliment Pharmacol Ther 2002,16(3):527-32. 10.1046/j.1365-2036.2002.01165.x
Nista EC, Candelli M, Zocco MA, Cazzato IA, Cremonini F, Ojetti V, Santoro M, Finizio R, Pignataro G, Cammarota G, Gasbarrini G, Gasbarrini A: Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2005,21(10):1241-7. 10.1111/j.1365-2036.2005.02412.x
Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J: High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr 2007,119(11-12):372-8. 10.1007/s00508-007-0807-2
Keating GM, Scott LJ: Moxifloxacin: a review of its use in the management of bacterial infections. Drugs 2004,64(20):2347-77. 10.2165/00003495-200464200-00006
Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE: Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis 2000,32(1):81-5. 10.1080/00365540050164272
Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J: High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr 2007,119(11-12):372-8. 10.1007/s00508-007-0807-2
Kusters JG, Kuipers EJ: Antibiotic resistance of Helicobacter pylori. Symp Ser Soc Appl Microbiol 2001, 30: 134S-44S.
Gisbert JP, Fernández-Bermejo M, Molina-Infante J, Pérez-Gallardo B, Prieto-Bermejo AB, Mateos-Rodríguez JM, Robledo-Andrés P, González-García G: First-line triple therapy with levofloxacin for Helicobacter pylori eradication. Aliment Pharmacol Ther 2007,26(3):495-500. PMID: 17635384 [PubMed - indexed for MEDLINE]
Antos D, Schneider-Brachert W, Bästlein E, Hänel C, Haferland C, Buchner M, Meier E, Trump F, Stolte M, Lehn N, Bayerdörffer E: 7-day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter 2006,11(1):39-45. 10.1111/j.0083-8703.2006.00375.x
Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, Megraud F, Soussy CJ, Cambau E: Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents 2007,29(4):389-96. 10.1016/j.ijantimicag.2006.11.007
Bogaerts P, Berhin C, Nizet H, Glupczynski Y: Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter 2006,11(5):441-5. 10.1111/j.1523-5378.2006.00436.x
Glocker E, Stueger HP, Kist M: Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrob Agents Chemother 2007,51(1):346-9. 10.1128/AAC.00614-06
Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D: Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liver Dis 2007,39(11):1001-5. 10.1016/j.dld.2007.06.016
Kumala W, Rani A: Patterns of Helicobacter pylori isolate resistance to fluoroquinolones, amoxicillin, clarithromycin and metronidazoles. Southeast Asian J Trop Med Public Health 2006,37(5):970-4. Erratum in: Southeast Asian J Trop Med Public Health. 2006, 37(6):1260.
Hung KH, Sheu BS, Chang WL, Wu HM, Liu CC, Wu JJ: Prevalence of primary fluoroquinolone resistance among clinical isolates of Helicobacter pylori at a University Hospital in Southern Taiwan. Helicobacter 2009,14(1):61-5. 10.1111/j.1523-5378.2009.00655.x
Bago J, Halle ZB, Strinić D, Kućisec N, Jandrić D, Bevanda M, Tomić M, Bilić A: The impact of primary antibiotic resistance on the efficacy of ranitidine bismuth citrate- vs. omeprazole-based one-week triple therapies in H. pylori eradication--a randomised controlled trial. Wien Klin Wochenschr 2002,114(12):448-53.
Acknowledgements
This study was funded in part by the Ministry of Science, Tehnology and Sports of the Republic of Croatia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This study was funded in part by the Ministry of Science, Tehnology and Sports of the Republic of Croatia.
I am an employee of General Hospital Sveti Duh.
I and my authors do not have any conflict of interest.
Prim.dr.sc. J. Bago, dr.med.
Authors' contributions
All authors have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bago, J., Majstorović, K., Belošić-Halle, Ž. et al. Antimicrobial resistance of H. pylori to the outcome of 10-days vs. 7-days Moxifloxacin based therapy for the eradication: a randomized controlled trial. Ann Clin Microbiol Antimicrob 9, 13 (2010). https://doi.org/10.1186/1476-0711-9-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-0711-9-13