Abstract
Background
The impact of outdoor air pollution on infant mortality has not been quantified.
Methods
Based on exposure-response functions from a U.S. cohort study, we assessed the attributable risk of postneonatal infant mortality in 23 U.S. metropolitan areas related to particulate matter <10 μm in diameter (PM10) as a surrogate of total air pollution.
Results
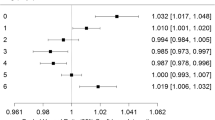
The estimated proportion of all cause mortality, sudden infant death syndrome (normal birth weight infants only) and respiratory disease mortality (normal birth weight) attributable to PM10 above a chosen reference value of 12.0 μg/m3 PM10 was 6% (95% confidence interval 3–11%), 16% (95% confidence interval 9–23%) and 24% (95% confidence interval 7–44%), respectively. The expected number of infant deaths per year in the selected areas was 106 (95% confidence interval 53–185), 79 (95% confidence interval 46–111) and 15 (95% confidence interval 5–27), respectively. Approximately 75% of cases were from areas where the current levels are at or below the new U.S. PM2.5 standard of 15 μg/m3 (equivalent to 25 μg/m3 PM10). In a country where infant mortality rates and air pollution levels are relatively low, ambient air pollution as measured by particulate matter contributes to a substantial fraction of infant death, especially for those due to sudden infant death syndrome and respiratory disease. Even if all counties would comply to the new PM2.5 standard, the majority of the estimated burden would remain.
Conclusion
Given the inherent limitations of risk assessments, further studies are needed to support and quantify the relationship between infant mortality and air pollution.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Research conducted during the last 10 to 20 years confirms that outdoor air pollution contributes to illness and death in adults and children [1]. The quantification of this public health problem and the benefits of its regulation, however, has been the subject of debates [2, 3]. Such impact assessments usually rely on extrapolations of data from various fields with partly unknown uncertainties [4], as it is rather rare to base the assessment on direct interventional observations. A few 'intervention studies', however, have been published, underlying the notion that air pollution causes morbidity and mortality, and that improvements in air quality consequently lead to direct health benefits [5–8]. So far, impact assessments have mostly focused on mortality in adults and a few measures of morbidity [2, 4, 9]. The impact assessment guidelines of WHO, however, emphasized the need for a broader assessment of the overal public health relevance [10, 11]. The update of the global burden of disease project of WHO, which quantified and ranked the health impact of 20 main risk factors, also included outdoor air pollution [12, 13], again with a strong focus on mortality. The burden of combustion-related urban air pollution in developed countries was estimated to exceed the impact of other considered environmental factors. In their study, Ezatti and colleagues estimated worldwide mortality due to urban outdoor air pollution for children under five years of age but did not provide disaggregated effects for infants [13]. We now estimated the burden of outdoor air pollution on infant mortality in selected areas of the U.S., where air pollution levels and infant mortality rates are relatively low and causes of infant deaths prevalent in developing countries are rare. To compare the underlying outcome frequencies and air quality aspects, we used only one U.S. study as a source to estimate the burden [14]. However, evidence is increasing for associations of outdoor air pollution with infant mortality and other adverse pregnancy outcomes [15–17].
Methods
We applied the risk assessment methods of a European study [4], which was adapted from others [18]. The method calculates attributable cases by using background rates of infant mortality (death register data), to which the epidemiological exposure-response function is applied [18]. This procedure leads to the number of attributable cases per unit of ambient air pollution concentration. We used particulate matter <10 μm in diameter (PM10) as a surrogate of air pollution mix. It is possible that the independent effects of other components (e.g. ozone or CO [19]) of air pollution may not be fully captured.
We used the exposure-response function from the only currently available cohort study in the U.S. conducted by Woodruff and colleagues based on approximately 4 million infants born between 1989–91 in 86 metropolitan areas [14]. Exposure was defined as the mean outdoor PM10 levels for the first two months of life. The authors controlled for some individual risk factors for infant mortality (i.e. – maternal education, maternal ethnicity, parental marital status, maternal smoking during pregnancy) and other potential confounders (i.e. – infant's month and year of birth, average temperature during first 2 months of life), and found that postneonatal mortality from all causes (excluding violent death) increased by 4% (95% confidence interval [CI] 2–7%) for every 10 μg/m3 PM10. Sudden infant death syndrome (SIDS) and respiratory disease mortality in infants with normal birth weight increased by 12% (95% CI 7–17%) and 20% (95% CI 6–36%) for every 10 μg/m3 PM10, respectively (Table 1, column 1). More recently, Lipfert and colleagues [20] reanalyzed these results, mostly confirming the PM10 associations; however, differences in study design and modeling did not allow including results from both of these studies in our risk assessment.
We applied the outcome-specific exposure-response functions from Woodruff and colleagues [14] to 25 counties in 23 U.S. metropolitan areas with a population of approximately 40 Million people and 700,000 infants born between 1995–97 who survived the neonatal period (Alabama: Jackson; California: Fresno, Los Angeles, Sacramento, San Diego, San Francisico; Colorado: Denver; Connecticut: Hartford; Illinois: Cook; Maryland: Baltimore; Michigan: Wayne; Missouri: St. Louis; New York: Bronx, Kings, New York; Pennsylvania: Philadelphia; Texas: El Paso, Harris, Dallas; Oklahoma: Oklahoma, Tulsa; Rhode Island: Providence; Utah: Salt Lake City; Washington: King; Wisconsin: Milwaukee). Criteria for the selection of counties were 1) that they were geographically distributed over the United States, 2) a population size above 500,000 and 3) availability of data on post-neonatal infant mortality and air pollution levels. We obtained annual mean outdoor PM10 levels for the years 1995–97 from the Environmental Protection Agency (EPA) air monitoring data and mortality rates for infants aged 1–12 months for the years 1995–97 from the National Center for Health Statistics. We did not quantify attributable cases for PM10 levels below 12.0 μg/m3 to avoid extrapolation beyond the lowest exposure level observed in Woodruff and colleagues [14]. Attributable mortality was calculated by county and then averaged across all counties. In another step we estimated the death burden of all counties that reached the new U.S. standard for particulate matter <2.5 μm in diameter (PM2.5) of 15 μg/m3(assuming an equivalent of 25 μg/m3 PM10).
Results
The mean PM10 level during 1995–97 was 28.4 μg/m3 (county range: 18.0 μg/m3 to 44.8 μg/m3). Fourteen of 25 counties had a 1995–97 mean PM10 level above 25 μg/m3. Across counties, all cause mean postneonatal infant mortality rates ranged from 130 to 352 per 100,000. Based on the risk assessment, postneonatal infant mortality rates and proportions attributable to PM10 air pollution are shown in Table 1. The estimated proportion of all cause mortality, SIDS (normal birth weight) and respiratory disease mortality (normal birth weight) attributable to PM10 above the reference level was 6% (95% CI 3–11%), 16% (95% CI 9–23%) and 24% (95% CI 7–44%), respectively. This estimate might be a reasonbable approximation of the attributable proportion across all metroplolitan areas of the U.S.. The expected number of infant deaths per year in the selected areas was 106 (95% CI 53–185), 79 (95% CI 46–111) and 15 (95% CI 5–27), repectively. For all outcomes, only a quarter of the burden was due to mean exposures above the new U.S. PM2.5 standard of 15 μg/m3 (equivalent to 25 μg/m3 PM10), whereas three quarters of the burden were due to PM10 levels between 12 μg/m3 PM10 (our reference level) and 25 μg/m3 PM10.
Discussion
Our risk assessment suggests that outdoor air pollution above a reference level of 12.0 μg/m3 PM10 contributes in a substantial way to postneonatal infant mortality. The estimated attributable proportions were particularly high for postneonatal mortality from SIDS and respiratory disease in infants born with a normal birth weight. The estimated contribution between the reference level and the new PM2.5 standard of 15 μg/m3 (equivalent to 25 μg/m3 PM10) revealed that the majority of the estimated burden would remain even if all counties would comply to the new PM2.5 standard. The results emphasize the need to address the diseases burden on a local and national level rather then at the global scale since risk factors that are rare in the U.S. dominate the overall burden [13].
Environmental health risk assessments suffer from inherent limitations related to uncertainties of the assumptions made [4]. Given the advantage of the cohort approach [21] and the potential limitations of geographic extrapolation, we relied on only one single albeit large U.S. study. In a previous risk assessment [4], we used the only two cohort studies available at the time for adult mortality [22, 23]. Selection criteria defined in the previous assessment, including adequate study design and published PM10 levels, were met.
A growing number of studies conducted in different countries using different study designs are showing effects of air pollution on infant mortality [24–27] and mortality in children under five years of age [28, 29]. Using previously reported methods [30], we were able to convert effects from all these studies except one [27] to relative risks per μg/m3 PM10. Effects ranged from 1.07 (95% CI 1.02–1.15) ([26], 3 day lag model) to 1.12 (95% CI 1.04–1.20) ([26], 3 day moving average model) for all cause infant mortality, 1.08 (95% CI 1.01–1.15) [25] to 1.09 (95% CI 1.03–1.14) [24] for postneonatal respiratory mortality, and 1.01 (95% CI 1.00–1.02) [29] to 1.02 (95% CI 1.01–1.02) [28] for mortality in children under five years of age. We have not attempted to use non-U.S. studies for a metaanalytic estimate because we believe that considerable differences in study regions do not allow transferring results. However, the effects found in these studies support the conclusion that the associations observed by Woodruff and colleagues reflect true effects of pollution, and are not just a result of uncontrolled confounding.
Other recent studies have focused on the impact of air pollution on fetal growth [31], preterm birth [19, 32, 33], birth weight [33–39], and other pregnancy outcomes such as smaller head circumference at birth [39] and cardiac malformations [19, 32]. Air pollution may have a harmful effect of lasting significance. Wilhelm and Ritz recently concluded from their research that compounds of motor vehicle exhaust may effect fetal development [33], potentially resulting in increased vulnerability of the respiratory and cardiovascular systems during infancy and childhood. Intrauterine growth retardation and low birth weight (LBW) have been linked to respiratory health later in life [40] and intrauterine growth retardation may lead to increased susceptibility to air pollution exposure and other environmental factors [41]. LBW may be on the causal pathway linking air pollution and infant mortality [20]. Furthermore, recent studies have shown growing evidence that ambient air pollution is associated with decreasing heart rate variability in adults [42]. The hypothezised underlying effect on the autonomic nervous system may also play a role in the genesis of SIDS [43]. There is clearly a need for further research to determine the impact of air pollution exposure on fetal development during pregnancy and to fill the gap of knowledge between our understanding of the underlying mechanisms and the observed associations of air pollution and mortality in infants.
We believe that the accumulating evidence justifies a risk assessment based on the assumption that ambient air pollution – with PM used as a surrogate for the mixture – causes infant deaths. Lipfert and colleagues [20] recently have questioned this assumption when they reanalyzed the association between air pollution and infant mortality using partly the same data as Woodruff and colleagues, because they observed unstable and partly inverse associations for some pollutants (albeit not for PM10). However, exposure was assigned on an annual base rather than accounting for the month of birth, and the stepwise multi-pollutant regression models used for the study is prone to artifacts.
Although we did not use the same metropolitan areas as Woodruff and colleagues, the size and extent of their study increased our confidence that the results are generalizable to other U.S. populations. Also, our results did not change considerably when we excluded counties in California, New York and Oklahoma that were not part of the original study. However, Woodruff and colleagues were not able to control for risk factors such as limited prenatal care or maternal age at delivery that may modify the effect estimates used in our assessment. In a more recent evaluation, Woodruff and colleagues have shown that mothers from different ethnic groups than caucasian were more likely to be exposed to high pollution levels and to have preterm delivery suggesting additional risk of residing in areas with poor air quality among infants at already increased risk of poor health [44]. Potential interactions with other county-specific factors, e.g. social deprivation, have been shown to be related to unknown factors that modify the effect of air pollution on mortality in adults [45, 46]. Exposure-response functions may be larger in such areas, with unpredictable influence on the overall impact. In our assessment, the total attributable risk based on population-weighting was higher than average across counties (7%, 95% CI 4–12% vs. 6%, 95% CI 3–11%), indicating that lack of control for risk factors in the original study may have resulted in an underestimate of the total effect. In addition, excluding infants with LBW may have resulted in increased social class homogeneity because low social class is a determinant of LBW [47], probably adding to an underestimate of the total effect estimated in our assessment.
Misclassification of causes of death is possible. It is, however, reassuring that the sum of the cause-specific attributable cases (14.0) closely corresponded to attributable cases based on the total mortality (14.7) estimate (Table 1).
Consistent with the underlying epidemiologic study, our assessment was based on fixed site monitors. For PM2.5 and PM10, current evidence suggests a high correlation between ambient and indoor concentrations from outdoor origin; thus, imprecision in exposure may be of minor concern for our assessment [22]. The choice of the exposure reference level is a large influence on the derived attributable cases [48]. Our assessment considered only the burden of concentrations higher then 12 μg/m3 (i.e. – the lowest observed level in the original study of Woodruff and colleagues). However, studies in adults give no evidence for a no-effect threshold even in time-series data with lowest concentrations being below 12 μ g/m3 [49]. Assuming an exposure reference level of 7.5 μg/m3 as used in other studies (e.g. [4, 13]) would result in an approximately 20–25% higher burden attributed to air pollution then the number of annual air pollution attributable cases for all cause infant mortality would increase from 106 (95% CI 53–185) to 132 (95% CI 66 to 232) if 7.5 μg/m3 was taken as the exposure reference level. We emphasize, however, that the shape of the risk function is less well defined in the lowest concentrations. Time-series mortality studies conducted with adults give no indication of a threshold of no effect, but this aspect has not been exploited for infant mortality. Thus, the burden derived for levels above 7.5 μg/m3 carries larger uncertainties than the one shown in our main results, where cases attributed to ambient levels below 12 μg/m3 are ignored. We also acknolwedge that the differentiation between an additive versus a multiplicative risk function has not been made in the Woodruff study nor in our risk assessment. However, given the very small relative risks and the application to only a limited range of exposure, this uncertainty is not of major concern.
Because it is generally not possible to assign effects of ambient air pollution to specific single pollutants, we considered PM as a surrogate measure of a more complex mixture. Part of the association between mortality and PM may be explained by the correlation of PM with other ambient air pollutants, and the estimated mortality may not be exclusively attributed to PM10. This uncertainty needs to be taken into account in the assessment of benefits of policies that target single pollutants rather than the mixture.
In case of mortality among adults, it may be preferable to convert the risk functions into estimates of the years of life lost [50]. For infants, we chose attributable cases as a preferred outcome. First, the measure can be directly derived from the published data, which did not measure life years lost. Second, some deaths may occurr among susceptible infants who may not have survived otherwise, a feature that has been called 'harvesting' in studies conducted with adults [51]. Although premature death of highly frail subjects plays only a minor role in short-term effects of air pollution among adults, the issue has not been addressed yet for infants, thus the uncertainty regarding time lost is particularly large. Third, the question arises whether attributable cases are preventable, given improved air quality. Quasi-experimental studies suggest fast short-term health benefits of clean air interventions for morbidity in children [52] or mortality in adults [6], but no data on infant mortality are available. As competing risks may become relevant after removal of one risk factor, the preventable fraction may be smaller. Although loss of life expectancy may be substantial among infant deaths [15], these are rare events in the developed world. Therefore, infant mortality due to air pollution can have only a minor effect on life expectancy in the U.S. population.
Conclusions
Evidence for a causal effect of air pollution on morbidity and mortality is strong for adults, and evidence is building that air pollution has an effect on infants and young children and a potential impact during the fetal period [19]. The evidence needs to be constantly reviewed as further studies become available. Our estimates are based on the best currently available information, leaving considerable uncertainty about the size of the true effect of particulate matter on infant mortality. However, given that the whole population is exposed, we conclude that air pollution-related infant mortality is a major public health problem. This outcome should be considered in future public health risk assessment and management and included in EPA's assessment of the benefits of the U.S. Clean Air Act [3].
Abbreviations
- CI:
-
confidence interval
- CO:
-
carbonmonoxyd
- EPA:
-
Environmental Protection Agency
- LBW:
-
low birth weight
- SIDS:
-
sudden infant death syndrome
- U.S.:
-
United States
- WHO:
-
World Health Organization
References
Brunekreef B, Holgate ST: Air pollution and health. Lancet. 2002, 360: 1233-1242. 10.1016/S0140-6736(02)11274-8.
EPA: Final report to Congress on Benefits and Costs of the Clean Air Act, 1970–1990. Environmental Protection Agency. Washington D.C., USA. 1997
NRC: Estimating Public Health Benefits of Proposed Air Pollution Regulation. National Research Council. Washington D.C., USA. 2002
Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, Herry M, Horak F, Puybonnieux-Texier V, Quenel P, Schneider J, Seethaler R, Vergnaud JC, Sommer H: Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000, 356: 795-801. 10.1016/S0140-6736(00)02653-2.
Heinrich MC, El-Rifai WM, Demetri G, Heinrich J: Improved air quality in reunified Germany and decreases in respiratory symptoms. Hum Pathol. 2002, 33: 466-477. 10.1053/hupa.2002.124122.
Clancy L, Goodman P, Sinclair H, Dockery DW: Effect of air-pollution control on death rates in Dublin, Ireland: an intervention study. Lancet. 2002, 360: 1210-1214. 10.1016/S0140-6736(02)11281-5.
Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM: Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001, 164: 2067-2072.
Friedman MS, Powell KE, Hutwagner L, Graham LM, Teague WG: Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA. 2001, 285: 897-905. 10.1001/jama.285.7.897.
EPA: Public hearing to consider amendments to the ambient air quality standards for particulate matter and sulfates. California Environmental Protection Agency, Air Resources Board. El Monte, CA, USA. 2002
WHO: Quantification of the Health Effects of Exposure to Air Pollution. A Report of a WHO Working Group. European Centre for Environment and Health. Copenhagen, Denmark. 2001
Krzyzanowski M, Cohen A, Anderson R: Quantification of health effects of exposure to air pollution. J Occup Environ Med. 2002, 59: 791-793. 10.1136/oem.59.12.791.
WHO: The World Health Report 2002. Reducing risks, promoting healthy life. World Health Organization. Geneva, Switzerland. 2002
Ezzati M, Lopez AD, Rodgers A, Vanderhoorn S, Murray CJL: Selected major risk factors and global and regional burden of disease. Lancet. 2002, 360: 1347-1360. 10.1016/S0140-6736(02)11403-6.
Woodruff TJ, Grillo J, Schoendorf KC: The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspect. 1997, 105: 608-612.
Brunekreef B: Air pollution kills babies...[editorial]. Epidemiology. 1999, 10: 661-662.
Romieu I, Samet JM, Smith KR, Bruce N: Outdoor air pollution and acute respiratory infections among children in developing countries. J Occup Environ Med. 2002, 44: 640-649.
Sram R: Impact of air pollution on reproductive health. Environ Health Perspect. 1999, 107: A542-543.
Krzyzanowski M: Methods for assessing the extent of exposure and effects of air pollution. Occup Environ Med. 1997, 54: 145-151.
Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA: Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002, 155: 17-25. 10.1093/aje/155.1.17.
Lipfert FW, Zhang J, Wyzga RE: Infant mortality and air pollution: a comprehensive analysis of U.S. data for 1990. J Air Waste Manag Assoc. 2000, 50: 1350-1366.
Kunzli N, Medina S, Kaiser R, Quenel P, Horak F, Studnicka M: Assessment of deaths attributable to air pollution: should we use risk estimates based on time series or on cohort studies?. Am J Epidemiol. 2001, 153: 1050-1055. 10.1093/aje/153.11.1050.
Dockery DW: An association between air pollution and mortality in six U.S. cities. N Eng J Med. 1993, 329: 1753-1759. 10.1056/NEJM199312093292401.
Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW: Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995, 151: 669-674.
Bobak M, Leon DA: Air pollution and infant mortality in the Czech Republic, 1986–88. Lancet. 1992, 340: 1010-1014. 10.1016/0140-6736(92)93017-H.
Bobak M, Leon DA: The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology. 1999, 10: 666-670. 10.1097/00001648-199911000-00001.
Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH: Air pollution and infant mortality in Mexico City. Epidemiology. 1999, 10: 118-123. 10.1097/00001648-199903000-00003.
Penna ML, Duchiade MP: Air pollution and infant mortality from pneumonia in the Rio de Janeiro metropolitan area. Bull Pan Am Health Organ. 1991, 25: 47-54.
Ostro B, Chestnut L, Vichit-Vadakan N, Laixuthai A: The impact of particulate matter on daily mortality in Bangkok, Thailand. J Air Waste Manag Assoc. 1999, 49: 100-107.
Conceicao GM, Miraglia SG, Kishi HS, Saldiva PH, Singer JM: Air pollution and child mortality: a time-series study in Sao Paulo, Brazil. Environ Health Perspect. 2001, 109 (Suppl 3): 347-350.
Dockery DW, Pope CA: Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994, 15: 107-132. 10.1146/annurev.pu.15.050194.000543.
Dejmek J, Selevan SG, Benes I, Solansky I, Sram RJ: Fetal growth and maternal exposure to particulate matter during pregnancy. Environ Health Perspect. 1999, 107: 475-480.
Ritz B, Yu F, Chapa G, Fruin S: Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000, 11: 502-511. 10.1097/00001648-200009000-00004.
Wilhelm M, Ritz B: Residential proximity to traffic and adverse birth outcomes in Los Angeles County, California, 1994–1996. Environ Health Perspect. 2003, 111: 207-216.
Bobak M, Richards M, Wadsworth M: Air pollution and birth weight in Britain in 1946. Epidemiology. 2001, 12: 358-359. 10.1097/00001648-200105000-00018.
Wang X, Ding H, Ryan L, Xu X: Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997, 105: 514-520.
Bobak M: Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000, 108: 173-176.
Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC: Is air pollution a risk factor for low birth weight in Seoul?. Epidemiology. 2001, 12: 643-648. 10.1097/00001648-200111000-00011.
Ritz B, Yu F: The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ Health Perspect. 1999, 107: 17-25.
Perera FP, Rauh V, Tsai WY: Effects of transplacental exposure to environmental pollutants on birth outcomes in a multethnic population. Environ Health Perspect. 2003, 111: 201-205.
Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST: Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999, 160: 227-236.
Ashworth A: Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998, 52 (Suppl 1): S34-42.
Aslan O, Goldeli O, Guneri S, Badak O, Fetil E, Ozkan S, Gold DR: Ambient pollution and heart rate variability. Can J Cardiol. 2000, 16: 345-351.
Patzak A: Short-term rhythms of the cardiorespiratory system and their significance in neonatology. Chronobiol Int. 1999, 16: 249-268.
Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC: Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003, 111: 942-946.
Krewski D: Reanalysis of the Harvard Sic Cities Study and the American Cancer Society Study of particulate air pollution and mortality. Health Effects Institute. Cambridge, MA, USA. 2000
Pope CA, Burnett TR, Thun MJ, Calle EE, Krewski D, Kazuhiko I, Thurston GD: Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002, 287: 1132-1141. 10.1001/jama.287.9.1132.
Spencer N, Bambang S, Logan S, Gill L: Socioeconomic status and birth weight: comparison of an area-based measure with the Registrar General's social class. J Epidemiol Community Health. 1999, 53: 495-498.
Kunzli N: The public health relevance of air pollution abatement. Eur Respir J. 2002, 20: 198-209. 10.1183/09031936.02.00401502.
Schwartz J: Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Am J Epidemiol. 2000, 151: 440-448.
Miller BG, Hurley JF: Life table methods for quantitative impact assessments in chronic mortality. J Epidemiol Community Health. 2003, 57: 200-206. 10.1136/jech.57.3.200.
Schwartz J: Harvesting and long term exposure effects in the relation between air pollution and mortality. Am J Epidemiol. 2000, 151: 440-448.
Heinrich J, Hoelscher B, Wichmann HE: Decline of ambient air pollution and respiratory symptoms in children. Am J Respir Crit Care Med. 2000, 161: 1930-1936.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
RK contributed to methods development, performed the analysis, reviewed the literature and wrote the manuscript.
IR reviewed part of the literature and contributed to methods development and writing.
SM, JS and MK contributed to methods development.
NK reviewed part of the literature and contributed to methods development and writing.
All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kaiser, R., Romieu, I., Medina, S. et al. Air pollution attributable postneonatal infant mortality in U.S. metropolitan areas: a risk assessment study. Environ Health 3, 4 (2004). https://doi.org/10.1186/1476-069X-3-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-069X-3-4