Abstract
Background
Control of the Anopheline mosquito vectors of malaria by use of insecticides has been shown to impact on both morbidity and mortality due to this disease. Evidence of insecticide resistance in different settings necessitates surveillance studies to allow prompt detection of resistance should it arise and thus enable its management. Possible resistance by Anopheles arabiensis mosquitoes from Mwea rice irrigation scheme in Central Kenya to insecticides in the four classes of insecticides approved by WHO for indoor residual spraying was investigated.
Methods
Susceptibility to DDT (an organochlorine), fenitrothion (an organophosphate), bendiocarb (a carbamate), lambdacyhalothrin and permethrin (both pyrethroids) was tested using standard WHO diagnostic bioassay kits. Bioassays were performed on non-blood fed mosquitoes one- to three-day old. Knockdown was recorded every 10 min and mortality 24 h post-exposure was noted.
Results
Mortality 24 h post-exposure was 100% for all insecticides except for lambdacyhalothrin, which averaged 99.46%. Knockdown rates at 10 min intervals were not significantly different between the Mwea population and the susceptible KISUMU strain of Anopheles gambiae sensu stricto control. The KDT50 and KDT95 values for the Mwea population were either lower than those for the control or higher by factors of no more than 2 for most comparisons and compared well with those of An. gambiae sensu lato categorized as susceptible in other studies.
Conclusion
These results suggest that the Mwea population of An. arabiensis is susceptible to all the insecticides tested. This implies that vector control measures employing any of these insecticides would not be hampered by resistance.
Similar content being viewed by others
Background
Anopheles gambiae sensu stricto, Anopheles arabiensis and Anopheles funestus are the most important vectors of malaria in sub-Saharan African and occur in sympatry across most of their range [1]. Studies show that the use of insecticides both for Indoor Residual Spraying (IRS) programmes and in the treatment of bed nets has resulted not only in a reduction in vector population densities but also in morbidity and mortality due to malaria [2–4]. There is, however, evidence that malaria vectors are developing resistance to commonly used insecticides [5]. In Western Kenya, resistance was first reported in the context of Insecticide-Treated Net (ITN) use [6]. Although more recent studies indicate that resistance levels have increased only marginally [7], there is concern that continued and/or increased use of insecticides may result in increased resistance that would threaten the sustainability of this vector control strategy. Insecticide resistance is more widespread in West Africa where it has been associated with use of insecticides in public health for mosquito control and in agriculture for pesticide control [8–11]. Levels of insecticide resistance have been shown to vary even within relatively small geographical scales and during different seasons [9, 10]. The dominant resistance mechanisms also vary as was observed in Guatemalan populations of Anopheles albimanus, where both insecticide resistance levels and mechanism varied within short distances [12]. These observations suggest the shifting nature of insecticide resistance and imply therefore that extrapolations from one circumstance to another may be misleading. Studies in Haitian populations of An. albimanus found resistance frequencies to fenitrothin to increase from 20 to 60% over a period of six months [13] and underscore the need for continuous insecticide resistance monitoring, even where no evidence of resistance has previously been found.
The current study presents the first report on the status of insecticide resistance/susceptibility in a rice-irrigation scheme in Central Kenya. Resistance was tested against insecticides in each of the four classes that have been approved for IRS by WHO. The results of this study will enable informed selection of insecticides for vector control programmes as well as provide baseline information essential in the monitoring of the development of insecticide resistance.
Materials and methods
Study area and insecticide use patterns
The study was carried out in Mwea area (00° 67'S, 37° 35'E) of Central Kenya. This is predominantly a rice-growing area although other crops such as beans, maize and green vegetables are grown for subsistence. Previously, rice was grown during a single growing season that extended from June to December but in recent years, different paddies are flooded intermittently during the year due to water shortages associated with the prevailing drought, thus maintaining almost all-year-round rice growing although the main growing season is still from June to December.
A survey to establish insecticide/pesticide-use patterns in the study area was conducted. This was done by administering a simple questionnaire on the pesticides used in agriculture and their concentrations and whether residents used bed nets and if they did, whether the bed nets were insecticide-treated. A total of 42 households were surveyed.
Specimen collection, identification and rearing
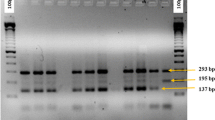
Specimens were collected both as larvae from rice paddies using standard dippers and as adults by aspiration from walls inside human dwellings. Collections were made on 4th and 5th August 2004 and again on 9th and 10th September 2004 during the dry season, which coincided with the main rice growing season and most paddies were flooded, and then again during the rainy season between 3rd – 5th May 2005. Specimens were identified as An. gambiae s.l. based on morphological characteristics [1]. Larvae from the different paddies were preserved live in separate bottles and transported to the insectary for rearing. The larvae were then reared into adults as follows: a single larval specimen was picked from each of the transportation bottles and placed in a rearing pan so that each pan contained just one specimen from each rice paddy. This was done to limit the chances that siblings were included in individual bioassay runs and thus obtain better estimates of population variability in insecticide susceptibility. Six pans were constituted in this manner and the resulting adults used for each of the five insecticide bioassays that were run and the sixth for the control test using untreated test paper. For the specimens collected as adults, individual field-collected females were allowed to oviposit and F1 families raised separately. Only one specimen from each of the families was used in each of the bioassays. Specimens were identified further to sibling species of the An. gambiae complex using species-specific Polymerase Chain Reaction technique [14] after DNA extraction by the alcohol precipitation method [15]. Field-collected adults were identified after they had oviposited while specimens collected as larvae were identified after the insecticide resistance bioassays were performed.
Insecticide susceptibility bioassays
Insecticide susceptibility assays were performed on adult non-blood fed mosquitoes one- to three-day old that were reared from field-collected larvae as described above or on F1s of field-collected adult mosquitoes. The tests were carried out using 4% DDT, 1% fenitrothion, 0.1% bendiocarb, 0.05% lambdacyhalothrin and 0.75% permethrin, the diagnostic doses recommended by WHO. The Bioassay kit, Mosquito (Adult) Diagnostic test kit WHO/VBC/81.806, was supplied by Universiti Sains Malaysia (USM), Penang, Malaysia and the assay carried out according to the accompanying instructions. Briefly, for each of the insecticides tested, mosquitoes were divided into batches between 15–25 mosquitoes and exposed to insecticide-treated papers for 1 h for DDT, bendiocarb and permethrin and for 2 h fenitrothion and lamdacyhalothrin. Insecticide knockdown effects were recorded every 10 min until 100% knockdown was observed. At the end of the exposure period, mosquitoes were transferred into tubes with untreated papers and allowed a 24 h recovery period after which mortality was recorded. Tests were accompanied by control tests where mosquitoes were exposed to papers treated only with silicone oil for 1 h or 2 h depending on the insecticide that was being tested against. Bioassays were also carried out on the An. gambiae KISUMU susceptible strain (KSM Strain). Mortality was noted 24 h post exposure as defined in the criteria for determining resistance or susceptibility to diagnostic doses of insecticide. All mosquitoes were supplied with a 6% glucose meal during the 24 h recovery period.
Statistical analyses
Mean mortality was determined across all batches of mosquitoes tested for a particular insecticide and the WHO [5] criteria used to evaluate the resistance/susceptibility status of the mosquito tested. By the said criteria, resistance is indicated by mortality rates of less than 80% 24 h after exposure to insecticide while mortality rates greater than 98% are indicative of susceptibility.
Mortality rates between 80–90% suggest the possibility of resistance that needs to be clarified. Knockdown rates at 10 min intervals for the Mwea larval and adult collections for each of the insecticides tested and for the dry and rainy season collections were compared using the paired t- test. Knockdown rates at 10 min intervals were also compared between the Mwea mosquito collections and the KSM strain using the paired t-test. Fifty and 95% knockdown times (KDT50 and KDT95 respectively) for both the Mwea collection and the KSM strain were estimated by the log-time probit model using the LdP LineR software [16]. The fit of the probit model was assessed using chi-square distribution analysis and the Bonferroni Procedure used to determine the overall significance of multiple tests.
Results
All households interviewed said that they had used fenitrothion as a pesticide in rice growing for at least the last ten years but did not know the concentration at which it was used. An interview with a manager at the Mwea Rice Growers Multipurpose Co-operative Society, the organization that supplies the pesticides to the farmers and through which the farmers sell their produce revealed that fenitrothion alongside carbofuran have been the pesticides in use for agricultural spraying but the use carbofuran was stopped two years prior to the study due to cost factors. Fenitrothion 50 EC is used at a concetration of 0.5% and is sprayed onto two-week old rice seedlings in the nursery and again 21–28 days after transplanting. The survey also revealed that no organized vector control programmes are available in the study area but that approximately 93% of the 42 households surveyed used bed nets. Of the total number of bed nets used, 39% were pyrethroid (deltamethrin)-treated but bed nets were not retreated after purchase. Approximately 55% of household also use either pyrethroid aerosol sprays or mosquito coils. The use of the aerosols and mosquito coils is higher during the rainy and the rice-growing season when the residents perceive that mosquito densities are high and thus also the threat of malaria.
The total numbers of field-collected specimens that were tested for each of the five insecticides are shown in the table. In addition, a total of 821 mosquitoes belonging to 49 families (family size 10–73 mosquitoes) were tested for susceptibility to lambdacyhalothrin after initial results indicated recovery after the 24 h period. All specimens tested were An. arabiensis by the specific-specific PCR assay.
Mortality, after the 24 h recovery period, was 100% for DDT, fenitrothion, bendiocarb and permethrin and for lambdacyhalothrin for the adult collection. Mortality was however slightly reduced for the lambdacyhalothrin assay with the larval collection and with the single-family samples, mortality being 99.1% ± 0.63 S.E.(for a total of 221 mosquitoes tested in 11 batches) and 99.3% ± 0.36 S.E. (for a total of 821 mosquitoes tested in 34 batches) respectively. Mortality on the KSM strain control was 100% for all the insecticides tested except for DDT (see Table 1).
Percentage knockdown at 10 min intervals was not significantly different between the Mwea larval and adult collections for all insecticides tested except for bendiocarb (paired t = 2.9896, df = 4, P = 0.0404). Data for the larval and adult assays were therefore merged for all comparisons except for this insecticide for all subsequent analyses. Percentage knockdown rates at 10 min intervals were also not significantly different between the dry and the rainy season collections nor between the Mwea collections and the KSM Strain for each of the insecticides tested (paired t, P > 0.05 in all cases). Figure 1 shows percentage knockdown versus exposure time for the Mwea collections and for the KSM Strain for each of the insecticides tested. The log-time probit model used to estimate KDT50 and KDT95 values did not fit the distribution of percentage knockdown with time for the fenitrothion assay for the Mwea collections or for the KSM Strain for the fenitrothion, lambdacyhalothrin and permethrin bioassays (P values for the chi-square test of heterogeneity <0.05 in each of these cases; P values were also significant for the global test). The KDT50 and KDT95 estimates in these cases were not therefore included in the comparisons as these would be unreliable although they are given in the table. All other KDT50 and KDT95 values for the Mwea population were either lower than those for the KSM Strain or only increased slightly, by factors of less than two.
Percentage knockdown against time for the Mwea An.arabiensis population and the An. gambiae KISUMU strain. The figure shows the results of insecticide resistance bioassays using diagnostic doses of each of the insecticides. Results are for mean knockdown across all batches of mosquitoes that were tested for each of the different seasons.
Discussion
Overall, the results obtained in this study suggest good susceptibility of An. arabiensis in the study area to all the five insecticides tested. This means that vector control programmes employing any of these compounds either in the treatment of bed nets or other materials or for indoor residual spraying would achieve satisfactory success rates. This is especially important as An. arabiensis was the only member of the An. gambiae complex found in the study area, a finding consistent with earlier studies in the area by researchers who found this species to constitute 87.3% of all Anopheline mosquitoes collected [17].
Based on the WHO criteria for characterizing insecticide resistance/susceptibility, where susceptibility is defined by mortality rates greater than 98% 24 h post-exposure, no evidence for resistance to any of the insecticides tested was found. Knockdown rates at 10 mins intervals were not significantly different between the Mwea collections and the KSM susceptible strain. In addition, KDT50 and KDT95 observed in the present study compare well with those from other studies for An. gambiae s.l. populations that are categorized as susceptible [9, 18, 19]. It is interesting to note that despite the high level of compliance and long-term use of fenitrothion, the An. arabiensis mosquito population has not developed resistance to this chemical. A possible explanation is that its levels in agricultural use are below what would select for possible naturally occurring resistance in this species.
The zero or near-zero levels of insecticide resistance in An. arabiensis that were observed in the present study are similar to those recently reported from an area of long-term ITN use in Western Kenya based on the presence of the knockdown resistance (kdr) gene [7]. The kdr mechanism results from mutations in the voltage-gated sodium channel, the target-site for DDT and pyrethroids and is one of the two most important forms of biochemical resistance mechanisms, the other being metabolic resistance, which occurs when levels of insecticide-detoxifying enzymes are elevated or their activity modified [20]. Similarly low or no resistance to pyrethroid insecticides and DDT caused by the kdr mutation has been observed within the M form of An. gambiae s.s. and An. arabiensis in several West African countries despite significant levels of resistance being found within the S form of An. gambiae s.s. [8, 9, 18–21]. The situation was however found to be different in South Africa where significant levels of resistance to DDT in An. arabiensis, by the WHO [5] criteria, were observed [22]. Earlier studies in the Sudan also found significantly high resistance levels to malathion in An. arabiensis [23], suggesting that this species is not immune to the development of resistance. These differences re-emphasize the focal nature of insecticide resistance and the need to carry out situation analyses and monitoring for individual settings. In Western Kenya, for example, the frequency of the kdr gene was found to increase albeit marginally four years after the introduction of ITNs but remained unchanged in villages 20 km away [7]. Studies to assess the effect of longer-term use of the ITNs on resistance in this area are crucial. In Burkina Faso, resistance levels were found to vary not only between villages within 100 km of each other and between different seasons but also to different insecticides, with resistance being seen to DDT but not to permethrin [9]. The Western Kenya and most of the West African studies, however, assayed only for the presence of the kdr gene to the exclusion of other possible resistance mechanisms. It would be interesting to obtain data on the levels of phenotypic resistance comparison. Brogdon and McAllister [20] have however argued that for insecticide resistance to be a concern, the level of resistance must be high enough to compromise the efficacy of intervention programmes employing the insecticides for vector control. It is controversial though what such a level would be given that studies in Côte d'Ivoir, for example, found nets impregnated with permethrin or deltamethrin to provide good levels of protection where the frequency of the kdr allele was 94% kdr [24].
Conclusion
These findings suggest that the An. arabiensis populations from Mwea are susceptible to all the insecticides that were tested against and therefore that vector control effort utilizing any of these insecticides would not be compromised by resistance. Thus, the results obtained in this study will enable informed choice of insecticides for use in vector control programmes in the area. In addition, the data obtained will provide baseline information needed in the monitoring of the development of resistance to the insecticides arising either due to selective pressure from the use of insecticides and pesticides or through migration to the area of mosquitoes with insecticide resistance genes.
References
Gillies MT, De Meillon B: The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). Publication of the South Africa Institute of Med Res. 1968, Johannesburg, 54: 203-207.
Mabaso ML, Sharp B, Lengeler C: Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004, 9: 846-856. 10.1111/j.1365-3156.2004.01263.x.
Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW: Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003, 68: 121-127.
Gimnig JE, Kolczak MS, Hightower AW, Vulule JM, Schoute E, Kamau L, Phillips-Howard PA, ter Kuile FO, Nahlen BL, Hawley WA: Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg. 2003, 68: 115-120.
WHO: Vector resistance to insecticides. 15th Report of the WHO Expert Committee on Vector Biology and Control. World Health Organ Tech Rep Ser. 1992, 818: 1-62.
Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW: Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994, 8: 71-75.
Stump AD, Atieli FK, Vulule JM, Besansky NJ: Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004, 70: 591-596.
Yawson AE, McCall PJ, Wilson MD, Donnelly MJ: Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol. 2004, 18: 372-377. 10.1111/j.0269-283X.2004.00519.x.
Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, Brengues C, Guillet P, Hemingway J, Small GH, Hougard JM: The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002, 67: 617-622.
Diabate A, Brengues C, Baldet T, Dabire KR, Hougard JM, Akogbeto M, Kengne P, Simard F, Guillet P, Hemingway J, Chandre F: The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: genetic introgression and de novo phenomena. Trop Med Int Health. 2004, 9: 1267-1273. 10.1111/j.1365-3156.2004.01336.x.
Awolola TS, Brooke BD, Koekemoer LL, Coetzee M: Absence of the kdr mutation in the molecular 'M' form suggests different pyrethroid resistance mechanisms in the malaria vector mosquito Anopheles gambiae s.s. Trop Med Int Health. 2003, 8: 420-422. 10.1046/j.1365-3156.2003.01034.x.
Brogdon WG, Beach RF, Stewart JM, Castanaza L: Microplate assay analysis of the distribution of organophosphate and carbamate resistance in Guatemalan Anopheles albimanus. Bull World Health Organ. 1988, 66: 339-346.
Brogdon JH, Hobbs WG, St Jean Y, Jacques JR, Charles LB: Microplate assay analysis of reduced fenitrothion susceptibility in Haitian Anopheles albimanus. J Am Mosq Control Assoc. 1988, 4: 152-158.
Scott JA, Brogdon WG, Collins FH: Identification of a single specimen of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993, 49: 520-529.
Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V: A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987, 37: 37-41.
Ehabsoft. [http://www.ehabsoft.com/ldpline]
Ijumba JN, Mwangi RW, Beier JC: Malaria transmission potential of Anopheles mosquitoes in the Mwea-Tebere irrigation scheme, Kenya. Med Vet Entomol. 1990, 4: 425-432.
Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF: Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003, 17: 326-332. 10.1046/j.1365-2915.2003.00449.x.
Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, Guillet P: Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull World Health Organ. 1999, 77: 230-234.
Brogdon WG, McAllister JC: Insecticide resistance and vector control. Emerging Infectious Diseases. 1998, 4 (4):
Fanello C, Petrarca V, della Torre A, Santolamazza F, Dolo G, Coulibaly M, Alloueche A, Curtis CF, Toure YT, Coluzzi M: The pyrethroid knock-down resistance gene in the Anopheles gambiae complex in Mali and further indication of incipient speciation within An. gambiae s.s. Insect Mol Biol. 2003, 12: 241-245. 10.1046/j.1365-2583.2003.00407.x.
Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M: Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003, 17: 417-422. 10.1111/j.1365-2915.2003.00460.x.
Hemingway J: Biochemical studies on malathion resistance in Anopheles arabiensis from Sudan. Trans R Soc Trop Med Hyg. 1983, 77: 477-480. 10.1016/0035-9203(83)90118-9.
Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P: Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae. Med Ve Entomol. 2000, 14: 81-88. 10.1046/j.1365-2915.2000.00212.x.
Acknowledgements
We thank J.N. Mwangi, L.N. Wachira and G. Gikandi for assistance in fieldwork and insectary work. This investigation received financial assistance from UNICEF/UNDP/World Bank/WHO Special Programme for Research and Technical Training in Tropical Diseases (TDR) Grant No. A30345. This paper is published with the permission of the Director, Kenya Medical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
LK conceived and designed the study, carried out the insecticide resistance bioassays, data analysis and interpretation and prepared the manuscript. JV participated in the development of the study design, carried out interpretation of the data and provided a critical review of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kamau, L., Vulule, J.M. Status of insecticide susceptibility in Anopheles arabiensis from Mwea rice irrigation scheme, Central Kenya. Malar J 5, 46 (2006). https://doi.org/10.1186/1475-2875-5-46
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-5-46