Abstract
Background
The evolution and spread of pyrethroid resistance in Anopheles gambiae s.s, the major malaria vector in sub-Saharan Africa, is of great concern owing to the importance of pyrethroid-treated nets in the WHO global strategy for malaria control. The impact of kdr (the main pyrethroid-resistance mechanism) on the behaviour of An. gambiae is not well understood. The objective of this study was to determine whether high or low doses of permethrin differ in their resistance-selection effects.
Methods
The effect of permethrin treatment was assessed under laboratory conditions using the tunnel test technique against susceptible, heterozygous and homozygous genotypes. Experimental huts trials were then carried out in Benin to assess the level of personal protection conferred by nets treated with a variety of permethrin concentrations and their impact on the selection for kdr allele.
Results
Tunnel tests showed that nets treated with permethrin at 250 and 500 mg/m2 induced higher mortality and blood feeding reduction among susceptible and heterozygous (RS) females as compared to the lower concentration (100 mg/m2). The experimental hut trials showed that the best personal protection was achieved with the highest permethrin concentration (1,000 mg/m2). Mosquito genotyping revealed a non-linear relationship in the survival of kdr susceptible and resistant genotypes with permethrin dosage. Higher dosages (≥250 mg/m2) killed more efficiently the RS genotypes than did lower dosages (50 and 100 mg/m2).
Conclusion
This study showed that nets treated with high permethrin concentrations provided better blood feeding prevention against pyrethroid-resistant An. gambiae than did lower concentrations. Permethrin-treated nets seem unlikely to select for pyrethroid resistance in areas where the kdr mutation is rare and present mainly in heterozygous form.
Similar content being viewed by others
Background
Pyrethroids are the only insecticides currently recommended by the World Health Organization for treatment of mosquito nets owing to their strong insecticidal activity at low concentrations and their low mammalian toxicity [1]. Pyrethroid-treated nets are effective in reducing malaria morbidity and mortality [2–4] and may also provide community protection through mass impact on vector mosquito populations, when used at a high coverage rate [5, 6].
Pyrethroid resistance in Anopheles gambiae has become widespread in different regions of Africa [7–9] and may represent a threat for successful and sustainable implementation of insecticide-treated net (ITN) programmes. There is evidence that massive use of DDT against cotton pests in the 1960s and 1970s was responsible for the selection of the kdr mutation (knock-down resistance) responsible for resistance to pyrethroids [8]. A point mutation (leucine to phenylalanine) in the S6 transmembrane segment domain II in the sodium channel sequence is associated with the kdr resistance in West Africa [10] and is characterized by a reduction in the intrinsic sensitivity of the insect nervous system to DDT and pyrethroids [11]. A second point mutation (leucine to serine) has also been reported in An. gambiae from East Africa and is responsible for high level of resistance to permethrin [12].
The selection of insecticide resistance is a complex evolutionary process [13] that may depend on genetic, ecological and operational factors [14]. Since operational factors are the main ones that can, in principle, theoretically be controlled, Georghiou [15] characterized resistance management tactics according to the intensity of exposure (low versus high doses), the frequency of applications and their use either in space or time. Because resistant genes are mainly present in a heterozygous state when they first evolve, a strategy to delay the development of resistance would involve doses which preferentially kill heterozygotes (RS) as well as susceptible homozygotes (SS) [16]. A good understanding of the response to insecticides by mosquitoes heterozygous for resistance is essential to understand how resistance may evolve and to develop tactics for resistance management.
In the present study, investigations were carried out on the behaviour and selection of An. gambiae of different genotypes for kdr exposed to nettings treated with a range of permethrin concentrations. Experiments were carried out under laboratory conditions using tunnel tests [17] and, in the field, using experimental huts [18].
Materials and Methods
Mosquitoes
Two laboratory strains of An. gambiae were used for the tunnel tests: A susceptible reference strain (Kisumu) of the S molecular form and a pyrethroid-resistant strain VKPR of the M molecular form [19], homozygous for the kdr mutation [20] with a resistance factor of ≈40 fold by topical application [21]. This resistant strain does not show additional resistance mechanisms to insecticides. In addition, hybrids (F1 progeny obtained from SS females and RR males crossing) were tested to evaluate the phenotypic expression of Kdr at heterozygous state.
Permethrin 10% Emulsifiable Concentrate (Peripel®) was applied at various concentrations to nets. For tunnel tests, pieces of netting (25 × 25 cm) (100 denier multi-filament polyester, meshes 156) were treated at 100, 250 and 500 mg/m2 taking care to ensure an even distribution of the insecticide. Tests were made 5 days after treatment in order to avoid deposits of heterogeneous ages, which might interfere with mosquito behaviour. Single size nets (11 m2) made of the same material were used for the experimental hut trial. Twelve nets were treated with permethrin at 50, 100, 250, 500 and 1,000 mg/m2 and untreated nets served as control. Each treated net was soaked for 5 minutes in diluted formulation at the desired concentration, gently wrung and laid horizontally in the shade for drying. Each net was deliberately holed (two rows of 90 holes of 4 cm2 cut along lines at 20 and 30 cm from the lower edge) to simulate badly torn nets allowing access for mosquitoes and to put emphasis insecticide treatment to protect sleepers.
Tunnel tests
Laboratory tests were performed in a square glass tunnel (height 25 cm, width 21 cm, length 60 cm) with cage ends, as described by Elissa & Curtis [17], subdivided by a changeable piece netting with 9 × 1 cm holes inserted on a cardboard frame across the tunnel. In one hand of the tunnel, a guinea pig was place as bait, held in a small metallic cage to prevent contact with the netting. In the other hand of the tunnel, 100 unfed female mosquitoes (5–8 days old) were introduced at 18.00 hours and the apparatus was left overnight in a dark room maintained at 28°c and 80% relative humidity. The next morning, at 08.00 hours, the numbers of mosquitoes in both compartments were counted and their mortality and blood feeding rates were scored. Tests were replicated three times for each genotype (SS, RS, RR), permethrin treatment (100, 250 and 500 mg/m2) and control untreated netting.
Experimental huts
The permethrin-treated bednets were tested in six experimental huts belonging to the CREC (Centre de Recherches Entomologiques de Cotonou, Benin). During the trial period (2 months from June to July), six adult men, who gave prior informed consent and received anti-malaria prophylaxis, slept under the nets in the experimental huts every night from 20.00 to 05.00 hours. To reduce variation in attractiveness to mosquitoes, sleepers were rotated between huts on successive nights. Nets were allocated to huts at random and used consistently in the same hut to avoid cross-contamination. Each net configuration (permethrin concentration) was duplicated and used every two nights in the same hut. Experimental hut trials were carried out according to Darriet et al. [22] and results expressed in terms of: mortality, blood feeding, deterrency (relative number of mosquitoes entering hut with treated nets) and induced exophily (relative proportion of mosquitoes collected outdoor in the veranda trap). An. gambiae females were stored individually with silica gel and genotyped for kdr [10] at LIN (Montpellier, France). Only 60% of females from the control hut were processed and all females from huts with treated nets were genotyped together with positive and negative controls. Species identification was confirmed by PCR using specific primers [23].
Statistical analysis
Mortality and blood feeding rates in tunnel tests were angular transformed (arc sin √x) to stabilize the variance. Two-way analysis of variance (ANOVA II) was carried out using the statistical software package "Statistica" (Stat-soft)R to determine effects of treatments, genotypes and their interactions. Experimental hut data were compared using Pearson chi-square test at 95% confidence interval. Conformity of kdr frequency with Hardy-Weinberg expectations was tested for each treatment (control, 50, 100, 250, 500 and 1,000 mg/m2) using the exact probability test based upon a null hypothesis of heterozygote excess or deficiency (GenopopR software) [24, 25].
Ethical approval
This study received a formal approval from the Ministry of Health of Benin and the Institut de Recherche pour le Développement.
Results
Tunnels tests
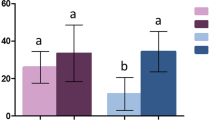
With untreated netting, between 45% to 70% of the females were able to feed on the guinea pig (Fig. 1). Dosage-dependent reduction in blood feeding were observed with SS and RS (P < 0.001). For RR females, no significant differences in the blood feeding rates were noted between treatments.
For the three genotypes, mortality rates ranged from 11 to 18% in the control tunnel (Fig. 2). On exposure to treated netting, the mortality rates for the SS females increased to 58% at 100 mg/m2 (P < 0.001) and to more than 80% at 250 and 500 mg/m2 (P < 0.0001). Mortality of RS females was generally intermediate between those of SS and RR, but was not statistically different at every concentration.
Experimental hut trials
A total of 839 An. gambiae females were collected in huts during the trial. PCR subsequently carried out confirmed that all An. gambiae were of the M molecular form [27, 28]. The allelic frequency for the kdr gene ranged from 63% to 78% between huts. A strong reduction in An. gambiae entry rates (80% to 90%) was observed in all huts fitted with treated nets, even at the lowest permethrin concentration (Table 1). This result confirmed that the deterrent effect of permethrin is maintained despite high kdr frequency [29]. This repellent effect was, however, not dosage-dependent. The low proportion of mosquitoes collected in the veranda-trap in the control hut (7%) was in accordance with the endophilic behaviour of An. gambiae. Induced exophily was greater for huts fitted with treated nets (P < 0.0001), especially at the higher dosage of 1,000 mg/m2 (about 90%).
All permethrin concentrations provided significant blood feeding reduction in comparison to control (P < 0.05). The best protection was achieved with the highest concentration (1,000 mg/m2), which reduced blood feeding by about 70%. Mortality rates in huts fitted with treated nets were low (17% to 25%) and not significantly different from the control (P > 0.05), except at the highest concentration. However, the unusually high mortality (27%) observed in the control hut (mainly in the veranda trap) may have masked a slight difference between control and treatments.
All concentrations of permethrin provided personal protection against pyrethroid-resistant Anopheles mosquitoes. The net treated with the highest permethrin concentration (1,000 mg/m2) showed significantly higher killing and excito-repellent effects and lower blood feeding than all other nets.
Permethrin concentrations and kdr genotypic distribution of Anopheles gambiae entering the huts (Table 2)
A total of 569 An. gambiae females were genotyped for kdr. The kdr genotypic frequencies were in accordance with Hardy-Weinberg expectations at each concentration and in the control (P > 0.05). The kdr frequency in the control hut was 68.5%, and ranged from 63.4% to 78.7% in the treatment huts. No significant difference was observed when comparing each control-treated pair (probability test, P > 0.05), except at 500 mg/m2 permethrin where the proportion of resistant homozygotes (RR) was slightly higher than in the control (P = 0.04). The genotypic distributions were not significantly different with lower concentrations (P > 0.05), indicating no selection of resistance genotypes.
Permethrin concentrations and kdr genotypic distribution of Anopheles gambiae found in the veranda-traps (Table 3)
All An. gambiae females collected in veranda traps were at Hardy-Weinberg equilibrium with regard to kdr (P > 0.05). The distributions of SS, RS and RR genotypes were similar for all treatments (P > 0.05). There was a deficit in RR and excess of RS in the control relative to treatments, but the result was not significant owing to low numbers in the veranda.
Permethrin concentrations and kdr genotypic distribution of Anopheles gambiae in relation with blood feeding (Table 4)
The kdr allelic frequencies were not significantly higher among blood-fed mosquitoes collected in huts fitted with treated nets than among unfed ones. However, significantly more homozygous resistant females took a blood meal in huts fitted with a net treated at 50 mg/m2 (P = 0.03) and 500 mg/m2 (P = 0.04) as compared to the control. The fact that only RS and RR genotypes were found among blood-fed females at 1,000 mg/m2, suggested that the kdr allele may have allowed females to stay longer on the treated netting, thus increasing chances to take a blood meal.
Permethrin concentrations and kdr genotypic distribution of Anopheles gambiae in relation with mortality (Table 5)
The kdr allelic frequency was not significantly higher among surviving mosquitoes than among dead ones (P > 0.05). However, the proportion of RS genotypes among dead mosquitoes was significantly greater (P = 0.01) at the highest concentration. No SS genotype was found among dead mosquitoes at 250, 500 and 1,000 mg/m2, suggesting that SS mosquitoes tend to avoid contact with nets treated at higher permethrin concentrations.
Discussion
In this tunnel test study, the response of An. gambiae females heterozygous for kdr to permethrin treated nets was comparable to that of susceptible ones, confirming that kdr is recessive in An. gambiae [21]. However, a previous study showed a greater efficacy of permethrin treated nets, at the same concentrations, against a pyrethroid-resistant strain of An. gambiae [21]. These authors demonstrated that resistant mosquitoes, which could tolerate higher doses of permethrin, stayed longer than susceptible ones on the treated surfaces and took up more insecticide than did susceptibles, resulting in a good efficacy of the pyrethroid treated nettings. Such contrasting results may be explain by larger number of holes in our netting screens of the tunnel tests (9 holes instead of 5), which allowed the resistant mosquitoes to make less contact with the impregnated material and resulted in lower mortality of RR females, even at the highest dose (500 mg/m2). This dosage, however, was the most efficient in killing preferentially RS and SS females. The experimental hut study showed that nets treated with 1,000 mg/m2 permethrin were the most effective against pyrethroid-resistant An. gambiae. However, permethrin concentration as high as 1,000 mg/m2 should not be recommended without careful consideration of cost and safety issues.
It was also demonstrated that there was little or no correlation between the permethrin concentration and the distribution of kdr genotypes as far as deterrency, excito-repellency, blood feeding and mortality were concerned. Interestingly, the kdr allelic frequency was not significantly different between mosquitoes which survived or died after exposure to permethrin treated nets, even at high concentrations. Such results contrasted with findings from other authors [30] who reported that nets treated either with alpha-cypermethrin or etofenprox in Côte d'lvoire, selected for kdr in An. gambiae. This difference could be attributed either to the chemical structure of the insecticide (permethrin being a non α-cyano-pyrethroid) or to differences in the excito-repellent effects of other pyrethroids. Excito-repellency is probably an important factor in the selection of pyrethroid resistance as it affects the duration of exposure of females to impregnated materials.
When An. gambiae females were exposed to nets treated with permethrin at the concentration recommended by WHO (250 to 500 mg/m2), the heterozygous ones (RS) were more efficiently killed than the susceptible ones (SS). Since kdr resistance to the irritant effect appeared to be co-dominant while resistance to lethal effect was recessive [21], the heterozygotes stayed longer than susceptible ones on the treated netting, picking up more insecticide and being killed in higher proportion. As a consequence, one can expect that permethrin treated nets are unlikely to select for pyrethroid resistance in areas where the kdr mutation is rare and mostly present in the heterozygous state.
Although other studies in West Africa have consistently shown that pyrethroid treated nets remain effective against kdr resistant An. gambiae [29, 31, 32], it is not possible to predict how long this effectiveness would be maintained should other resistance mechanisms appear. With indoor residual spraying in South Africa, an increased level of mixed function oxidase activity in Anopheles funestus was associated with severe malaria control failure [33]. The current spread of pyrethroid resistance in the major malaria vectors An. gambiae and An. funestus emphasizes the need to identify alternative insecticides and for the development and implementation of effective and sustainable resistance management strategies. Other experimental huts studies suggested that non-pyrethroid insecticides, such as organophosphates or carbamates, have potential for use on mosquito nets [34, 35]. These chemicals are less excito-repellent than pyrethroids, and allow for a longer contact between mosquito and insecticide treated netting's, inducing higher mortality [36]. Unfortunately, a cross resistance to both carbamates and organophospshorous insecticides involving an insensitive acetylcholinesterase has recently been detected in An. gambiae from Côte d'lvoire [37, 38] and may, therefore, impede their interest for ITNs in the concerned areas. Furthermore, recent evidence showed that carbosulfan-treated nets (300 mg/m2) does select for resistance in the An. gambiae population from the same area [39], unlike pyrethroids with respect to kdr. In the absence of new alternative insecticides for treatment of mosquito nets, the possible use of mixtures of insecticides or mosaic treatments [40–42] should be closely investigated, as a possible strategy to maintain the effectiveness of ITNs and prevent the development of resistance. In areas where resistance genes are already present (Kdr and Ace.1R), ITNs are still the best option. Despite the presence of insensitive acetylcholinesterase at significant level conferring resistance to carbamates and organophosphates [37], nets treated with these insecticides (carbosulfan and pirimiphos methyl respectively) were very effective in killing resistant mosquitoes and, in the case of carbosulfan, were also effective in preventing blood feeding [35–40]. Recent studies have shown that another organophosphate (chlorpyrifos-methyl) is also effective and potentially safe enough to be considered as a possible alternative, either alone or in combination, for treatment of mosquito nets [43, 44]. In contrast, in areas where malaria control is based on indoor residual spraying, resistance may lead to failure of control operations, although this is not yet confirmed in areas with kdr or insensitive AChE resistance.
Conclusions
This study showed that pyrethroid-treated nets still remain effective in pyrethroid-resistance area and do not have a significant impact on selection of kdr mutation in An. gambiae. Such findings provide useful information for malaria vector control programme given that kdr resistance is now widely distributed in malaria vectors in Africa.
Author's contributions
VC undertook the insecticide tests and wrote the paper. CB carried out the mosquito genotyping. JMH and FL participated in the drafting and revising of the manuscript. PG and FC designed the study and made critical comments in the paper. MA supervised the ITNs evaluation in the field.
References
Zaim M, Aitio A, Nakashima N: Safety of pyrethroid-treated mosquito nets. Med Vet Entomol. 2000, 14: 1-5. 10.1046/j.1365-2915.2000.00211.x.
Greenwood BM, Baker JR, editors: A malaria control trial using insecticide treated bed nets and targeted chemoprophylaxis in a rural area of the Gambia, West Africa. A review of the epidemiology and control of malaria in The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1993, 87: 3-11. 10.1016/0035-9203(93)90169-Q.
Choi HW, Breman JG, Teutsh SM, Liu S, Hightower AW, Sexton JD: The effectiveness of insecticide-impregnated bed nets in reducing cases of Malaria infection. A meta-analysis of published results. Am J Trop Med Hyg. 1995, 52: 377-382.
Lengeler C, Cattani J, De Savigny D: Net Gain. Operational Aspects of a New Health Intervention for Preventing Malaria. World Health Organization. 1996, IDRC and Geneva
WHO: A Global Strategy for Malaria Control. World Health Organization. 1993, Geneva
Takken W: Do insecticide-treated bednets have an effect on malaria?. Trop Med Int Hlth. 2002, 7: 1022-1030. 10.1046/j.1365-3156.2002.00983.x.
Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW: Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994, 8: 71-75.
Chandre F, Darriet F, Manga L, Akogbeto M, Faye O, Mouchet J, Guillet P: Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull World Health Organ. 1999, 77: 230-234.
Etang J, Manga L, Chandre F, Guillet P, Fondjo E, Mimpfoundi R, Toto JC, Fontenille D: Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera: Culicidae) in the Republic of Cameroon. J Med Entomol. 2003, 40: 491-497.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D: Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998, 7: 179-184. 10.1046/j.1365-2583.1998.72062.x.
Pauron D, Barhanin J, Amichot M, Pralavorio M, Bergé JB, Lazdunski M: Pyrethroid receptor in the insect NA + channel: alteration of its properties in pyrethroid-resistant flies. Biochemistry. 1989, 28: 1673-1677.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH: Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000, 9: 491-497. 10.1046/j.1365-2583.2000.00209.x.
Rosenheim JA, Tabashnik BE: Evolution of pesticide resistance: interactions between generation time and genetic, ecological, and operational factors. J Econ Entomol. 1990, 83: 1184-1193.
Georghiou GP, Taylor CE: Pesticide resistance as an evolutionary phenomenon. In Proceedings of the 15th International Congress of Entomology. 1976, Washington DC, America, ESO (ed). Entomological Society of America, 759-785.
Georghiou GP: Principles of insecticide resistance management. Phytoprotection. 1994, 75: 51-59.
Curtis CF, Miller JE, Hodjati MH, Kolaczinski JH, Kasumba I: Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors?. Philos Trans R Soc Lond Bio Sci: Biological Sciences. 1998, 353: 1769-1775. 10.1098/rstb.1998.0329.
Elissa N, Curtis CF: Evaluation of different formulations of deltamethrin in comparison with permethrin for impregnation of netting. Pestic Sci. 1995, 44: 363-367.
Darriet F, N'Guessan R, Hougard JM, Traoré-Lamizana M, Carnevale P: Un outil expérimental indispensable à l'évaluation des insecticides: les cases-pièges. Bull Soc Pathol Exot. 2002, 95: 299-303.
Chandre F, Manguin S, Brengues C, Dossou-Yovo J, Darriet F, Diabate A, Carnevale P, Guillet P: Current distribution of a pyrethroid resistance gene (Kdr) in Anopheles gambiae complex from West Africa and further evidence for reproductive isolation of the mopti form. Parassitologia. 1999, 41: 319-322.
Darriet F, Guillet P, Chandre F, N'Guessan R, Doannio JMC, Rivière F, Carnevale P: Présence et évolution de la résistance aux pyréthrinoïdes et au DDT chez deux populations d'Anopheles gambiae d'Afrique de 1'Ouest. World Heath Organization WHO/CTD/VBC/97.1001, WHO/MAL/97.1081. 1997, 15-
Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P: Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae. Med Vet Entomol. 2000, 14: 81-88. 10.1046/j.1365-2915.2000.00212.x.
Darriet F, Vien NT, Robert V, Carnevale P: Evaluation of the efficacy of permethrin impregnated intact and perforated mosquito nets against vectors of malaria. World Health Organization WHO/VBC/84899, WHO/MAL/84.1008. 1984
Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993, 49: 520-529.
Raymond M, Rousset F: Genepop (version 1.2), a population genetics software for exact tests and ecumenicism. J Heredity. 1995, 86: 248-249.
Rousset F, Raymond M: Testing heterozygotes excess and defiency. Genetics. 1995, 140: 1413-1419.
Goudet J, Raymond M, De Meeüs T, Rousset F: Testing differentiation in diploid population. Genetics. 1996, 144: 1933-1940.
Weill M, Chandre F, Brengues C, Manguin S, Akogbéto M, Pasteur N, Guillet P, Raymond M: The Kdr mutation occurs in the Mopti form of Anopheles gambiae s.s. through introgression. Insect Mol Biol. 2000, 9: 451-455. 10.1046/j.1365-2583.2000.00206.x.
Fanello C, Akogbeto M, Della Torre A: Distribution of the pyrethroid knockdown resistance gene (Kdr) in Anopheles gambiae s.l from Benin. Trans R Soc Trop Med Hyg. 2000, 94: 132-
Darriet F, Guillet P, N'Guessan R, Doannio JMC, Koffi AA, Konan LY, Carnevale P: Impact de la résistance d'Anopheles gambiae s.s. à la perméthrine et à la deltaméthrine sur l'efficacité des moustiquaires imprégnées. Méd Trop (Mars). 1998, 58: 349-354.
Fanello C, Kolaczinski JH, Conway DJ, Carnevale P, Curtis CF: The kdr pyrethroid resistance gene in Anopheles gambiae: tests of non-pyrethroid insecticides and a new detection method for the gene. Parassitologia. 1999, 41: 323-326.
Guillet P: Implications of the Knock-down resistance (kdr) development on the use of impregnated bednets. In Proceedings of 1st Meeting of Global Collaboration for Development of Pesticides for Public Health, Geneva CTD/WHOPES/GCDP/98.1. 45-48. 14–15 October 1998
Darriet F, N'Guessan R, Koffi AA, Konan L, Doannio JMC, Chandre F, Carnevale P: Impact of pyrethrin resistance on the efficacy of impregnated mosquito nets in the prevention of malaria: results of tests in experimental cases with deltamethrin SC. Bull Soc Pathol Exot. 2000, 93: 131-134.
Hargreaves K, Koerkemoer LL, Brooke B, Hunt RH, Mthembu J, Coetzee M: Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000, 14: 181-189. 10.1046/j.1365-2915.2000.00234.x.
Miller JE, Lindsay SW, Armstrong JRM: Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticides for mosquito control in The Gambia. Med Vet Entomol. 1991, 5: 465-467.
Kolaczinski JH, Fanello C, Herve JP, Conway DJ, Carnevale P, Curtis CF: Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bull Entomol Res. 2000, 90: 125-132.
Curtis CF, Mnzava AEP: Comparison of house spraying and insecticide-treated nets for malaria control. Bull World Health Organ. 2000, 78: 1389-1400.
N'Guessan R, Darriet F, Guillet P, Carnevale P, Traore-Lamizana M, Corbel V, Koffi AA, Chandre F: Resistance to carbosulfan in field populations of Anopheles gambiae from Côte d'lvoire based on reduced sensitivity of acetylcholinesterase. Med Vet Entomol. 2003, 17: 19-25.
Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, Pasteur N, Philips A, Fort P, Raymond M: Insecticide resistance in mosquito vectors. Nature. 2003, 423: 136-137. 10.1038/423136b.
Corbel V, Hougard JM, N'Guessan R, Chandre F: Evidence for selection of insecticide resistance due to insensitive acetylcholinesterase by carbamate treated nets in Anopheles gambiae s.s. (Diptera: Culicidae) from Côte d'lvoire. J Med Entomol. 2003, 40: 985-988.
Guillet P, N'Guessan R, Darriet F, Traoré-Lamizana M, Chandre F, Carnevale P: Combined pyrethroid and carbamate "two in one" treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med Vet Entomol. 2001, 15: 105-112. 10.1046/j.1365-2915.2001.00288.x.
Corbel V, Darriet F, Chandre F, Hougard JM: Insecticides mixtures for mosquito net impregnation against malaria vector. Parasite. 2002, 9: 255-259.
Hougard JM, Corbel V, N'Guessan R, Darriet F, Chandre F, Akogbéto M, Baldet T, Guillet P, Carnevale P, Traoré-Lamizana M: Efficacy of mosquito nets treated with insecticide mixtures and mosaics against insecticide resistant Anopheles gambiae and Culex quinquefasciatus in Côte d'lvoire. Bull Entomol Res. 2003, 93 (6): 491-498. 10.1079/BER2003261.
N'Guessan RN, Asidi A, Rowland MW, Curtis CF, Hougard JM, Corbel V, Lines JL, Zaim M: The organophosphorous insecticide chlorpyrifos-methyl restores the efficacy of insecticide treated nets in an area of pyrethroid resistant anopheline and culicine mosquitoes. In Proceedings of 52nd Annual Meeting and Centennial Celebration of the American Society of Tropical Medicine and Hygiene, Philidelphia, Pennsylvania, USA. 539-540. 3–7 December 2003
Darriet F, Corbel V, Hougard JM: Efficacy of mosquito nets treated with a pyrethroid-organophosphorous mixture against Kdr- and Kdr+ malaria vectors (Anopheles gambiae). Parasite. 2003, 10: 359-362.
Acknowledgements
We are very grateful to the staff of the Centre de Recherches Entomologiques de Cotonou for their support during the experimental hut study. Thanks also go to the sleepers in the huts for their participation in the present work. Permethrin was kindly provided by Agrevo (Berkhamsted, UK) and the mosquito nets by Siamdutch (Bangkok, Thailand).
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Corbel, V., Chandre, F., Brengues, C. et al. Dosage-dependent effects of permethrin-treated nets on the behaviour of Anopheles gambiae and the selection of pyrethroid resistance. Malar J 3, 22 (2004). https://doi.org/10.1186/1475-2875-3-22
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-3-22