Abstract
Background
Although the World Health Organization recommends replacing quinine (QN) by artesunate due to its increased efficacy and the higher tolerance to the drug in both adults and children, QN remains a first-line treatment for severe malaria, especially in Africa. Investigations of microsatellite Pfnhe-1 ms4760 polymorphisms in culture-adapted isolates from around the world have revealed that an increase in the number of DNNND amino acid motifs was associated with decreased QN susceptibility, whereas an increase in the number of DDNHNDNHNND motifs was associated with increased QN susceptibility.
Methods
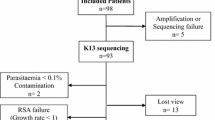
In this context, to further analyse associations between Pfnhe-1 ms4760 polymorphisms and QN susceptibility, 393 isolates freshly collected between October 2009 and January 2010 and July 2010 and February 2011, respectively, at the Hôpital Principal de Dakar, Senegal were assessed ex vivo for QN susceptibility, and their genes were amplified and sequenced.
Results
Of the 393 Plasmodium falciparum clinical isolates collected, 145 were successfully cultured. The 145 QN IC50s ranged from 2.1 to 1291 nM, and 17 isolates (11.7%) exceed the QN reduced susceptibility threshold of 611 nM. Among the 393 P. falciparum clinical isolates, 47 different alleles were observed. The three most prevalent profiles were ms4760-1 (no = 72; 18.3%), ms4760-3 (no = 65; 16.5%) and ms4760-7 (no = 40; 10.2%). There were no significant associations observed between QN IC50 values and i) the number of repeats of DNNND in block II (p = 0.0955, Kruskal-Wallis test); ii) the number of repeats of DDNHNDNHNND in block V (p = 0.1455, Kruskal-Wallis test); or iii) ms4760 profiles (p = 0.1809, Kruskal-Wallis test).
Conclusions
Pfnhe-1 ms4760 was highly diverse in parasite isolates from Dakar (47 different profiles). Three profiles (ms4760-1, ms4760-3 and ms4760-7) were predominant. The number of repeats for block II (DNNND) or block V (DDNHNDNHNND) was not significantly associated with QN susceptibility. New studies, and especially in vivo studies, are necessary to confirm the role of Pfnhe-1 ms4760 as a marker of QN resistance.
Similar content being viewed by others
Background
Although the World Health Organization (WHO) recommends replacing quinine (QN) with artesunate due to its improved efficacy and the higher tolerance of the drug in both adults and children [1, 2], QN remains a first-line treatment for severe malaria, especially in Africa, and is still used as a second-line therapy in combination with doxycycline, tetracycline or clindamycin for uncomplicated malaria in many countries [3]. Despite the efficacy of QN against chloroquine-resistant Plasmodium falciparum isolates, reports of QN resistance (QNR) have been increasing. In the 1980s, the frequency of clinical failures increased in Southeast Asia [4–6], South America [7] and Africa [8, 9]. Despite the longevity of QN use, the mechanisms of resistance (and its mode of action) have not yet been resolved. QN, a quinoline derivative, is a monoprotic weak base that accumulates within the low pH environment of the parasite digestive vacuole of P. falciparum. QN presumably acts by interference with the detoxification of haem produced during haemoglobin degradation by P. falciparum asexual blood stages [10]. The mechanism of QNR is complex and multigenic. QNR has been associated with mutations in both the P. falciparum multidrug resistance gene mdr1 (Pfmdr1) [11] and the chloroquine resistance transporter gene Pfcrt[12]. More recently, other genetic polymorphisms, such as mutations in the resistance protein gene Pfmrp[13], have been suggested to contribute to QNR. PfMRP knockout parasites displayed an increased susceptibility to QN [14]. Using quantitative trait loci (QTL) on the genetic cross of HB3 and Dd2 strains, Ferdig et al. identified genes associated with reduced QN susceptibility on chromosome 5, encoding Pfmdr1, on chromosome 7, encoding Pfcrt, and on chromosome 13, encoding the sodium/hydrogen exchanger gene Pfnhe-1[15]. Sequences of Pfnhe-1 showed multiple and complex variations including point polymorphisms at three separate codons (790, 894 and 950) and microsatellite variations in three different repeat sequences (msR1, ms3580 and ms4760). However, the three point polymorphisms and microsatellite polymorphisms msR1 and ms3580 showed no significant associations with QN susceptibility. Conflicting data have been reported on Pfnhe-1 polymorphisms. However, the investigations of the microsatellite ms4760 polymorphisms in culture-adapted isolates from around the world showed an association with the QN susceptibility phenotype [16]. A repetition of the amino acid motif DNNND was associated with a decreased susceptibility to QN based on the clinical failure of QN in a traveller from Senegal [17], and data from fresh isolates from Vietnam (n = 79) [18] and from culture-adapted isolates from the China-Myanmar border area (n = 60) [19], Asia, South America and Africa (n = 95) [20]. In 29 cultured-adapted isolates from the Kenya [21] and in 172 freshly obtained isolates from Uganda [22], the duplication of the DNNND motif was associated with a reduced susceptibility to QN compared to isolates with one or more than two repeats. Moreover, an increased number of DDNHNDNHNND motifs were associated with an increased susceptibility to QN [15, 16, 18–20]. Paradoxically, increased numbers of this latter amino acid motif were associated with a reduced susceptibility to QN based on 83 freshly obtained isolates from Madagascar and 13 African countries [23]. Moreover, these samples did not exhibit any associations between the number of DNNND repeats and QN susceptibility. Furthermore, there was no association between the number of DNNND and DDNHNDNHNND repeats and QN susceptibility based on freshly obtained isolates from the Republic of Congo (n = 74) [24], Thailand (n = 85) [25], Asia, South America and Africa(n = 90) [20].
In this context, to further analyse associations between polymorphisms in Pfnhe-1 ms4760 and QN susceptibility, 393 freshly obtained isolates from Dakar, Senegal were assessed ex vivo for QN susceptibility and their genes were amplified and sequenced.
Methods
Reference culture-adapted strains and clinical isolates of Plasmodium falciparum
Between October 2009 and January 2010 and July 2010 and February 2011, 393 P. falciparum clinical isolates were collected from patients with malaria recruited at the Hôpital Principal de Dakar, a military hospital, in the context of an evaluation of ex vivo malaria susceptibility to anti-malarial drugs in Dakar [26, 27]. Venous blood samples were collected in Vacutainer® ACD tubes (Becton Dickinson, Rutherford, NJ, USA) prior to patient treatment. Informed verbal consent was obtained from patients and/or their parents before blood collection. An assessment of P. falciparum susceptibility to anti-malarial drugs was performed using the same venous blood sample used for this diagnostic. The study was reviewed and approved by the ethical committee of the Hôpital Principal de Dakar.
Thin blood smears were stained using a RAL® kit (Réactifs RAL, Paris, France) and were examined to determine P. falciparum density and confirm monoinfection. Parasitized erythrocytes were washed three times with RPMI 1640 medium (Invitrogen, Paisley, UK) buffered with 25 mM HEPES and 25 mM NaHCO3. If parasitaemia exceeded 0.5%, infected erythrocytes were diluted to 0.5% with uninfected erythrocytes (human blood type A+) and re-suspended in RPMI 1640 medium supplemented with 10% human serum (Abcys S.A. Paris, France), for a final haematocrit of 1.5%.
Drugs
QN was purchased from Sigma (Saint Louis, MO, USA) and was dissolved first in methanol and then diluted in water to final concentrations ranging from 5 nM to 3200 nM. Batches of plates were tested and validated using the CQ-susceptible 3D7 strain (West-Africa) and the CQ-resistant W2 strain (Indochina) (MR4, Virginia, USA) in three to six independent experiments using the conditions described in the paragraph below. The two strains were synchronized twice with sorbitol before use [28], and clonality was verified every 15 days using PCR genotyping of the polymorphic genetic markers msp1 and msp2 and using microsatellite loci [29, 30] and additionally verified each year by an independent laboratory from the Worldwide Anti-malarial Resistance Network (WWARN).
Ex vivo assay
For in vitro isotopic microtests, 200 μl of synchronous parasitized red blood cells (final parasitaemia, 0.5%; final haematocrit, 1.5%) was aliquoted into 96-well plates pre-dosed with anti-malarial drugs. The plates were incubated in a sealed bag for 42 h at 37°C with the atmospheric generators for capnophilic bacteria Genbag CO2® at 5% CO2 and 15% O2 (BioMérieux; Marcy l’Etoile, France) [31]. After thawing the plates, haemolysed cultures were homogenized by vortexing the plates. Both the success of the drug susceptibility assay and the appropriate volume of haemolysed culture to use for each assay were determined for each clinical isolate during a preliminary pLDH ELISA. Both pre-test and subsequent experimental ELISAs were performed using a commercial kit (ELISA-Malaria antigen test, ref 750101, DiaMed AG, Cressier s/Morat, Switzerland) as previously described [32]. The optical density (OD) of each sample was measured with a spectrophotometer (Multiskan EX, Thermo Scientific, Vantaa, Finland).
The concentration at which the drugs were able to inhibit 50% of parasite growth (IC50) was calculated with the inhibitory sigmoid Emax model with an estimation of the IC50 through non-linear regression using a standard function of the R software (ICEstimator version 1.2) [33]. IC50 values were validated only if the OD ratio (OD at concentration 0 / OD at concentration max) was superior to 1.8 and the confidence interval ratio (upper 95% confidence interval of the IC50 estimation/lower 95% confidence interval of the IC50 estimation) was inferior to 2.0 [33].
Genotyping of the Pfnhe ms4760 microsatellite polymorphisms
Parasite DNA from 100 μl of infected blood was extracted using the E.Z.N.A. Blood DNA kit (Omega Bio-Tek, GA, USA). A sequence containing the previously described ms4760 microsatellite [15] was amplified using pfnhe-3802 F 5′-TTATTAAATGAATATAAAGA-3′ and pfnhe-4322R 5′-TTTTTTATCATTACTAAAGA-3′ primers. Sequencing was performed using ABI Prism Big Dye Terminator v1.1 Cycle Sequencing Ready Reaction Kits (Applied Biosystems, CA, USA), according to the manufacturer’s instructions. Sequences were analysed with BioEdit sequence alignment editor (version 7.0.9.0) software.
Statistical analysis
Data were analysed using R software (version 2.10.1). Differences between the QN IC50 values of isolates harbouring DNNND repeats, DDNHNDNHNND repeats or profiles were compared using the Kruskal-Wallis test.
Results
Of the 393 P. falciparum clinical isolates collected at the Hôpital Principal de Dakar, 145 isolates were successfully cultured. The 145 QN IC50s ranged from 2.1 to 1291 nM, and 17 isolates (11.7%) exceeded the QN-reduced-susceptibility threshold of 611 nM that has been previously defined [31].
Among the 393 P. falciparum clinical isolates, 47 different alleles were observed, including five profiles not previously described (from ms4760-109 to ms4760-113) (Figure 1). The amino acid sequence alignments of these 47 profiles are described in Figure 2. The three most prevalent profiles were ms4760-1 (no = 72; 18.3%), ms4760-3 (no = 65; 16.5%) and ms4760-7 (no = 40; 10.2%).
The number of repeats for block II (DNNND) ranged from zero to four, and the number of repeats for block V (DDNHNDNHNND) ranged from one to three (Figure 3). For block II, groups with one repeat (33.1%), two repeats (33.8%) and three repeats (29.5%) represented the majority of the 393 isolates. For block V, the group with two repeats alone represented 63.4% of the isolates.
There was no observed significant association between QN IC50 values and the number of repeats of DNNND in block II (p = 0.0955, Kruskal-Wallis test) (Table 1). The 145 isolates were classified into two groups: < 2 repeats of DNNND and ≥ 2 repeats. The QN IC50 values were not significantly different in the group < 2 repeats of DNNND in block II (mean IC50 = 184.1 nM, 95% confidence interval 134.6-252.4) compared with the group ≥ 2 repeats of DNNND in block II (mean IC50 = 153.5 nM, 95% confidence interval 123.3-191.0) (p = 0.2224, Kruskal-Wallis test).
There was no observed significant association between QN IC50 values and the number of repeats of DDNHNDNHNND in block V (p = 0.1455, Kruskal-Wallis test) (Table 2). The 145 isolates were classified into two groups: < 2 repeats of DDNHNDNHNND and ≥ 2 repeats. The QN IC50 values were not significantly different in the group < 2 repeats of DDNHNDNHNND in block V (mean IC50 = 134.0 nM,
95% confidence interval 95.9-187.1) in comparison to the group ≥ 2 repeats of DDNHNDNHNND in block V (mean IC50 = 175.8 nM, 95% confidence interval 142.2-217.3) (p = 0.1084, Kruskal-Wallis test).
Additionally, there was no observed significant association between QN IC50 values and ms4760 profiles (p = 0.1809, Kruskal-Wallis test) (Table 3).
Discussion
QN has been used to treat malaria for more than 350 years in Africa, with little emergence and spread of resistance. Although WHO recommends replacing QN with artesunate, QN remains the first-line anti-malarial treatment for complicated malaria in Europe and Africa. Although QN has retained good anti-malarial efficacy in most areas, its clinical efficacy has decreased in some regions. The first cases of QN clinical failure were observed in Brazil and Asia in the 1960s; then in the 1980s, clinical failures became more frequent in Southeast Asia, South America and Africa [4–9]. However, QN resistance is not yet a significant problem in Africa, and QN remains both the first-line drug used to treat severe malaria and a second-line therapy for uncomplicated malaria in some areas of Africa.
Even in areas where QN remains effective, such as sub-Saharan Africa, the susceptibility of individual P. falciparum isolates to QN has varied widely. The IC50s for isolates collected in Senegal were 31 to 765
nM in 1984 (Thies and Kaolack) [34], 5 to 932 nM in 1996 (Dielmo) [35] and 6 to 1291 nM in 2009 (Dakar) [26]. Many other studies have reported wide ranges of susceptibility to QN: 25 to 1253 nM in Comoros [36], 36 to 1097 nM in the Republic of Congo [24] (studies assessed in the same conditions as studies in Senegal in 1996 and 2009) or 15 to 761 nM in Uganda [22]. The wide range of QN susceptibility and recent evidence for QN treatment failure seen across Africa [8, 9, 17] suggest that the evolution of parasites with reduced susceptibility may contribute to decreased QN efficacy.
Although some reports of QN treatment failure exist, it is difficult to confirm QN resistance because of the drug’s short elimination half-life, the requirement to administer it three times a day for at least five days, drug intolerance that often leads to poor compliance and a lack of reliable data on the correlation between QN IC50 and clinical failure.
Even if WHO recommends replacing QN with artesunate as first-line anti-malarial treatment for complicated malaria, maximizing the efficacy and longevity of QN remains important and will depend critically on the pursuit of intensive research towards the identification of in vitro markers of QNR and the implementation of ex vivo and
in vivo surveillance programs, such as those championed by the WorldWide Antimalarial Resistance Network [37, 38]. Specifically, there is a need to identify molecular markers that effectively predict QN resistance and enable the active surveillance of temporal trends in parasite susceptibility [39]. The present study aimed to evaluate the association between the Pfnhe polymorphism and QN susceptibility in freshly obtained isolates from Dakar, Senegal to assess the validity of Pfnhe as a molecular marker of QN susceptibility in this region.
Prior to this report, no data were yet available on the sequence variation of Pfnhe-1 ms4760 in P. falciparum parasites from Senegal. Among the 393 studied sequences, 47 different alleles were observed in samples from Dakar. Consistent with previous reports [22, 24, 40], Pfnhe-1 ms4760 was highly diverse among parasite isolates. It appears that polymorphisms are more important in Africa and the Indian Ocean region than in India or Asia: in Senegal, 47 different profiles (393 samples) were observed; in the Republic of Congo, 27 different profiles (74 samples) [24]; in Uganda, 40 different profiles (172 samples) [22]; and in the Indian Ocean, 29 different profiles (595 samples) [40], whereas in Vietnam, only ten different profiles (79 samples) were observed [18]; in the China-Myanmar border area, ten different profiles (60 samples) [19]; and
in India, 16 different profiles (244 samples) [41]. This situation likely reflects the level of transmission in these areas and the level of QN selection pressure. The genetic diversity of ms4760, assessed by Nei’s unbiased expected heterozygosity (He), was significantly higher in African isolates (ranged from 0.66 to 0.85) and Indian isolates (0.68) than in Asian isolates (0.49 to 0.68) [40]. Only three profiles (ms4760-1, ms4760-3 and ms4760-7) of the four expected predominant profiles (ms4760-1, ms4760-3, ms4760-6 and ms4760-7) [18–24, 40, 41] were found to predominate in Senegal. Profile ms4760-6 represents only 1.8% of the studied sequences. The predominance of these three profiles combined with a low rate of the ms4760-6 profile was also found in parasites collected before QN treatment and in recurrent parasites in Mali [42].
The 145 QN IC50s ranged from 2.1 to 1291 nM and, in 17 isolates (11.7%), exceeded the QN-reduced-susceptibility threshold of 611 nM that has been previously defined [31]. In this study, the number of repeats for block II (DNNND) or block V (DDNHNDNHNND) was not significantly associated with QN susceptibility. These data are similar to those found in freshly obtained isolates from Asia, South America and Africa [20], the Republic of Congo [24] or Thailand [25]. The reduced susceptibility to QN was associated with an increased number of DNNND repeats or with two repeats, and the increased susceptibility to QN associated with an increased number of DDNHNDNHNND repeats was found more often in culture-adapted parasites [19–21]. Given that the influence of Pfnhe on QN susceptibility has been shown to be parasite-dependent, these apparently conflicting results may be explained, in part, by differences in the geographic origin of the parasites analysed, as their local selection history and genetic background varies, and by the method used to assess in vitro susceptibility to QN (i.e. an in vitro test for culture-adapted isolates or strains versus an ex vivo test for freshly obtained isolates) [20].
One explanation for these differences could be variation in genetic background. A specific genetic background observed in Asia may allow the observed contribution of Pfnhe polymorphism to QN in vitro susceptibility. This explanation is consistent with the following: i) the first evidence of Pfnhe-QN resistance association from QTL analysis using Americano-Asian cross strains [15], ii) that most associations identified have been shown among Asian strains [15, 16, 18, 19] and iii) that at least five genes spanning the P. falciparum genome influence the QNR in vitro phenotype with an additive effect or with pairwise interactions [15].
Additionally, these genetic dissimilarities between African and Asian Plasmodium populations may be accentuated by different local selection histories. The best-documented genomic modifications by a local selection process relate to drug pressure. Several studies have shown that drug pressure may involve extended linkage disequilibrium around a drug resistance associated gene [43]. This is characterized by a strong loss of genetic diversity called a selective sweep. This process stretch may be modified by either i) drug use or ii) malaria transmission level. A reduced drug use and higher malaria transmission level in Africa would be consistent with the lower selective sweep and an absence of linkage disequilibrium between Pfnhe and other cooperative drug response genes or selected compensatory mutations. For example, the QTL analysis of chromosome 13 located 60 genes of unknown function as being close to Pfnhe[15]. They would be more or less linked depending on drug pressure and malaria transmission levels if QN pressure selects at least one of them. In Kenya, there is an association between two DNNND repeats in the ms4760 Pfnhe microsatellite and a reduced susceptibility to QN in 29 P. falciparum isolates [44]. This is consistent with the historic precedent of the spread of drug resistance around the world. The emergence of chloroquine resistance in Asia was followed by an initial introduction into East Africa and subsequent spread across the African continent. Geographic proximity may explain plasmodial population migration. Moreover, East African plasmodial populations may exhibit genetic dissimilarities to other African populations [29].
As the QN response is controlled by multiple genes with complex interactions, one would expect: i) a higher sensitivity to genetic background than if the response were controlled by only one gene, ii) higher sensitivity to parameters that might cause linkage disequilibrium between genes and iii) various combinations of gene polymorphisms that might result in similar QN resistance phenotypes.
The three profiles (ms4760-1, ms4760-3 and ms4760-7) were predominant in parasites collected before QN treatment and in recurrent parasites in Mali [42]. The prevalence of ms4160-1 increased significantly from 26.2% to 46.3% after QN treatment in recurrent parasites. QN treatment selected for the Pfnhe-1 ms4760-1 profile. The profiles ms4760-3 and ms4760-18 were found in recurrent parasites from a patient returning from French Guiana and Senegal who failed with QN treatment [17, 45]. Such in vivo studies are necessary to confirm the role of Pfnhe-1 ms4760 as a marker of QN resistance. However, according to both previous studies and this current one, as Pfnhe-1 exhibits inconsistent association with QN susceptibility, the current problem should be to identify the new other marker that will be best associated with reduced QN susceptibility.
Conclusion
Pfnhe-1 ms4760 was highly diverse in parasite isolates in Dakar (47 different profiles). Genetic diversity should be assessed. Three profiles (ms4760-1, ms4760-3 and ms4760-7) were predominant. The number of repeats for block II (DNNND) and block V (DDNHNDNHNND) was not significantly associated with QN susceptibility. New studies, and especially in vivo studies, are necessary to confirm the role of Pfnhe-1 ms4760 as a marker of QN resistance. Studies should aim to identify partners of Pfnhe-1 or other polymorphisms linked to Pfnhe-1.
References
Dondorp A, Nosten F, Stepniewska K, Day N, White N, South-East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group: Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005, 366: 717-735.
Dondorp AM, Fanello CI, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Blay Nguah S, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Jonhson WBR, Tshefu AK, Onyamboko MA, Sakulthaew T: Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010, 376: 1647-1657. 10.1016/S0140-6736(10)61924-1.
World Health Organization: Guidelines for the treatment of malaria. 2010, Geneva, Switzerland: WHO, Second edition
Chongsuphajaisiddhi T, Sabchareon A, Attanath P: Treatment of quinine resistant falciparum malaria in Thai children. Southeast Asian J Trop Med Public Health. 1983, 14: 357-362.
Pukrittayakamee S, Supanaranond W, Looareesuwan S, Vanijanonta S, White NJ: Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans R Soc Trop Med Hyg. 1994, 88: 324-327. 10.1016/0035-9203(94)90102-3.
Harinasuta T, Bunnag D, Lasserre R: Quinine resistant falciparum malaria treated with mefloquine. Southeast Asian J Trop Med Public Health. 1990, 21: 552-557.
Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF: Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg. 1998, 58: 630-637.
Jelinek T, Schelbert P, Loscher T, Eichenlaub D: Quinine resistant falciparum malaria acquired in east Africa. Trop Med Parasitol. 1995, 46: 38-40.
Palmieri F, Petrosillo N, Paglia MG, Conte A, Goletti D, Pucillo LP, Menegon M, Sannella A, Severini C, Majori G: Genetic confirmation of quinine-resistant Plasmodium falciparum malaria followed by postmalaria neurological syndrome in a traveler from Mozambique. J Clin Microbiol. 2004, 42: 5424-5426. 10.1128/JCM.42.11.5424-5426.2004.
Hawley SR, Bray PG, Mungthin M, Atkinson JD, O’Neill PM, Ward SA: Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1998, 42: 682-686.
Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF: Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000, 403: 906-909. 10.1038/35002615.
Cooper RA, Lane KD, Deng B, Mu J, Patel JJ, Wellems TE, Su X, Ferdig MT: Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol Microbiol. 2007, 63: 270-282. 10.1111/j.1365-2958.2006.05511.x.
Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ: Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol Microbiol. 2003, 49: 977-989. 10.1046/j.1365-2958.2003.03627.x.
Raj DK, Mu J, Jiang H, Kabat J, Sing S, Sullivan M, Fay MP, McCutcham TF, Su XZ: Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drug and glutathione. J Biol Chem. 2009, 284: 7687-7696. 10.1074/jbc.M806944200.
Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE: Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol. 2004, 52: 985-997. 10.1111/j.1365-2958.2004.04035.x.
Henry M, Briolant S, Zettor A, Pelleau S, Baragatti M, Baret E, Mosnier J, Amalvict R, Fusai T, Rogier C, Pradines B: Plasmodium falciparum Na+/H + exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob Agents Chemother. 2009, 53: 1926-1930. 10.1128/AAC.01243-08.
Pradines B, Pistone T, Ezzedine K, Briolant S, Bertaux L, Receveur MC, Parzy D, Millet P, Rogier C, Malvy D: Quinine-resistant malaria in traveler returning from Senegal, 2007. Emerg Infect Dis. 2010, 16: 546-548. 10.3201/eid1603.091669.
Sinou V, Quang Le H, Pelleau S, Huong VN, Huong NT, Tai Le M, Bertaux L, Desbordes M, Latour C, Long LQ, Thanh NX, Parzy D: Polymorphism of Plasmodium falciparum Na(+)/H(+) exchanger is indicative of a low in vitro quinine susceptibility in isolates from Viet Nam. Malar J. 2011, 10: 164-10.1186/1475-2875-10-164.
Meng H, Zhang R, Yang H, Fan Q, Su X, Miao J, Cui L, Yang Z: In vitro sensitivity of Plasmodium falciparum clinical isolates from the China-Myanmar border area to quinine and association with polymorphism in the Na+/H + exchanger. Antimicrob Agents Chemother. 2010, 54: 4306-4313. 10.1128/AAC.00321-10.
Pelleau S, Bertaux L, Briolant S, Ferdig MT, Sinou V, Pradines B, Parzy D, Jambou R: Differential association of Plasmodium falciparum Na+/H + exchanger polymorphism and quinine responses in field- and culture-adapted isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 2011, 55: 5834-5841. 10.1128/AAC.00477-11.
Okombo J, Kiara SM, Rono J, Mwai L, Pole L, Ohuma E, Borrmann S, Ochola LI, Nzila A: In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium isolates from Kenya. Antimicrob Agents Chemother. 2010, 54: 3302-3307. 10.1128/AAC.00325-10.
Baliraine FN, Nsobya SL, Achan J, Tibenderama JK, Talisuna AO, Greenhouse B, Rosenthal PJ: Limited ability of Plasmodium falciparum pfcrt, pfmdr1, and pfnhe1 polymorphims to predict quinine in vitro sensitivity or clinical effectiveness in Uganda. Antimicrob Agents Chemother. 2011, 55: 615-622. 10.1128/AAC.00954-10.
Andriantsoanirina V, Menard D, Rabearimanana S, Hubert V, Bouchier C, Tichit M, Bras JL, Durand R: Association of microsatellite variations of Plasmodium falciparum Na+/H + exchanger (Pfnhe-1) gene with reduced in vitro susceptibility to quinine: lack of confirmation in clinical isolates from Africa. Am J Trop Med Hyg. 2010, 82: 782-787. 10.4269/ajtmh.2010.09-0327.
Briolant S, Pelleau S, Bogreau H, Hovette P, Zettor A, Castello J, Baret E, Amalvict R, Rogier C, Pradines B: In vitro susceptibility to quinine and microsatellite variations of the Plasmodium falciparum Na+/H + exchanger (pfnhe-1) gene: the absence of association in clinical isolates from the Republic of Congo. Malar J. 2011, 10: 37-10.1186/1475-2875-10-37.
Poyomtip T, Suwandittakul N, Sitthichot N, Khositnithikul R, Tan-Ariya P, Mungthin M: Polymorphisms of the pfmdr1 but not the pfnhe-1 gene is associated with in vitro quinine sensitivity in Thai isolates of Plasmodium falciparum. Malar J. 2012, 11: 7-10.1186/1475-2875-11-7.
Fall B, Diawara S, Sow K, Baret E, Diatta B, Fall KB, Mbate PS, Fall F, Diémé Y, Rogier C, Wade B, Bercion R, Pradines B: Ex vivo susceptibility of Plasmodium isolates from Dakar, Senegal, to seven standard anti-malarial drugs. Malar J. 2011, 10: 310-10.1186/1475-2875-10-310.
Wurtz N, Fall B, Pascual A, Diawara S, Sow K, Baret E, Diatta B, Fall KB, Mbaye PS, Fall F, Diémé Y, Rogier C, Bercion R, Briolant S, Wade B, Pradines B: Prevalence of molecular markers of Plasmodium falciparum drug resistance in Dakar. Senegal. Malar J. 2012, 11: 197-10.1186/1475-2875-11-197.
Lambros C, Vanderberg JP: Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979, 65: 418-420. 10.2307/3280287.
Bogreau H, Renaud F, Bouchiba H, Durand P, Assi SB, Henry MC, Garnotel E, Pradines B, Fusai T, Wade B, Adehossi E, Parola P, Kamil MO, Puijalon O, Rogier C: Genetic diversity and structure of African Plasmodium falciparum populations in urban and rural areas. Am J Trop Med Hyg. 2006, 74: 953-959.
Henry M, Diallo I, Bordes J, Ka S, Pradines B, Diatta B, M’Baye PS, Sane M, Thiam M, Gueye PM, Wade B, Touze JE, Debonne JM, Rogier C, Fusai T: Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am J Trop Med Hyg. 2006, 75: 146-151.
Pascual A, Basco LK, Baret E, Amalvict R, Travers D, Rogier C, Pradines B: Use of the atmospheric generators for capnophilic bacteria Genbag CO2® for the evaluation of in vitro Plasmodium falciparum susceptibility to standard anti-malarial drugs. Malar J. 2011, 10: 8-10.1186/1475-2875-10-8.
Kaddouri H, Nakache S, Houzé S, Mentré F, Le Bras J: Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother. 2006, 50: 3343-3349. 10.1128/AAC.00367-06.
Le Nagard H, Vincent C, Mentré F, Le Bras J: Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011, 104: 10-18. 10.1016/j.cmpb.2010.08.003.
Brandicourt O, Druilhe P, Diouf F, Brasseur P, Turk P, Danis M: Decreased sensitivity to chloroquine and quinine of some Plasmodium falciparum strains from Senegal in september 1984. Am J Trop Med Hyg. 1986, 35: 717-721.
Pradines B, Tall A, Parzy D, Spiegel A, Fusai T, Hienne R, Trape JF, Doury JC: In vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. J Antimicrob Chemother. 1998, 42: 333-339. 10.1093/jac/42.3.333.
Parola P, Pradines B, Simon F, Carlotti MP, Minodier P, Ranjeva MP, Badiaga S, Bertaux L, Delmont J, Morillon M, Silai R, Brouqui P, Parzy D: Antimalarial drug susceptibility and point mutations associated with resistance in 248 Plasmodium falciparum isolates imported from Comoros to Marseille. Am J Trop Med Hyg. 2007, 77: 431-437.
Sibley CH, Barnes KI, Plowe CV: The rationale and plan for creating a World Antimalarial Resistance Network (WARN). Malar J. 2007, 6: 118-10.1186/1475-2875-6-118.
Sibley CH, Barnes KI, Watkins WM, Plowe CV: A network to monitor antimalarial drug resistance: a plan for moving forward. Trends Parasitol. 2008, 24: 43-48. 10.1016/j.pt.2007.09.008.
Plowe CV, Roper C, Barnwell JW, Happi CT, Joshi HH, Mbacham W, Meshnick SR, Mugittu K, Naidoo I, Price RN, Shafer RW, Sibley CH, Sutherland CJ, Zimmerman PA, Rosenthal PJ: World Antimalarial Resistance Network (WARN) III: molecular markers for drug resistant malaria. Malar J. 2007, 6: 121-10.1186/1475-2875-6-121.
Andriantsoanirina V, Khim N, Ratsimbasoa A, Witkowsky B, Benedet C, Canier L, Bouchier C, Tichit M, Durand R, Ménard D: Plasmodium falciparum Na+/H + exchanger (pfnhe-1) genetic polymorphism in Indian Ocean malaria-endemic areas. Am J Trop Med Hyg. 2013, 88: 37-42. 10.4269/ajtmh.2012.12-0359.
Vinayak S, Tauqeer Alam M, Upadhyay M, Das MK, Dev V, Singh N, Dash AP, Sharma YD: Extensive genetic diversity in the Plasmodium falciparum Na+/H + exchanger 1 transporter protein implicated in quinine resistance. Antimicrob Agents Chemother. 2007, 51: 4508-4511. 10.1128/AAC.00317-07.
Kone A, Mu J, Maiga H, Beavoqui AA, Yattara O, Sagara I, Tekete MM, Traore OB, Dara A, Dama S, Diallo N, Kodio A, Traoré A, Bjorkman A, Gil JP, Doumbo OK, Wellems TE, Djimde AA: Quinine treatment selects pfnhe-1 ms4760-1 polymorphism in Malian patients with falciparum malaria. J Infect Dis. 2013, 207: 520-527. 10.1093/infdis/jis691.
Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ: Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002, 418: 320-323. 10.1038/nature00813.
Nkrumah LJ, Riegelhaupt PM, Moura P, Johnson DJ, Patel J, Hayton K, Ferdig MT, Wellems TE, Akabas MH, Fidock DA: Probing the multifactorial basis of Plasmodium falciparum quinine resistance: evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol Biochem Parasitol. 2009, 165: 122-131. 10.1016/j.molbiopara.2009.01.011.
Bertaux L, Kraemer P, Taudon N, Trignol A, Martelloni M, Saidi R, Parzy D, Pradines B, Simon F: Quinine-resistant malaria in traveller returning from French Guiana, 2010. Emerg Infect Dis. 2011, 17: 943-945. 10.3201/eid1705.101424.
Acknowledgments
The authors thank the patients of the Hôpital Principal de Dakar, Ndeye Fatou Diop and Maurice Gomis for technical support and the staff of the clinical units. This work was supported by the Etat Major des Armées Françaises (grant schema directeur paludisme LR 607).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AP and NW carried out the molecular genetic studies. BF, ER and BP carried out the ex vivo evaluation of QN susceptibility. MF, CC, AN, BD, KBF, PSM, YD, RB and BW supervised, carried out and coordinated the field collections of patient isolates. BP conceived and coordinated the study. SB, HB, CR and BP analysed the data. NW, AP, SB, HB, BF and BP drafted the manuscript. All the authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pascual, A., Fall, B., Wurtz, N. et al. In vitro susceptibility to quinine and microsatellite variations of the Plasmodium falciparum Na+/H+ exchanger transporter (Pfnhe-1) gene in 393 isolates from Dakar, Senegal. Malar J 12, 189 (2013). https://doi.org/10.1186/1475-2875-12-189
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-12-189