Abstract
Neurohumoral stimulation comprising both autonomic-nervous-system dysfunction and activation of hormonal systems including the renin-angiotensin-aldosterone system (RAAS) was found to be associated with Type-2-diabetes (T2D). Therapeutic strategies such as RAAS interference proved to be beneficial in both T2D treatment and prevention. In addition to an activated RAAS, hyperleptinemia in obesity, hyperinsulinemia in conditions of peripheral insulin resistance and overall oxidative stress in T2D represent known activators of the sympathetic component of the autonomic nervous system. Here, we hypothesize that sympathetic activation may cause peripheral insulin resistance defined as partial blocking of insulin effects on glucose uptake. Resulting hyperinsulinemia or hyperglycemia-related oxidative stress may further aggravate sympatho-excitation. This notion leads to a secondary hypothesis: sympathetic activation worsens from obesity towards insulin resistance, and further towards T2D. In this review, existing evidence relating to neurohumoral stimulation in T2D and consequences thereof, such as oxidative stress and inflammation, are discussed. The aim of this review is to provide a rationale for therapies, which are able to intercept neuroendocrine pathways in T2D and precursor states such as obesity.
Similar content being viewed by others
Background
Both Type-2-Diabetes (T2D) and Chronic Heart Failure (CHF) may be perceived as consequences of originally diverse pathologies. In the CHF condition, the diagnosis of ischemic or non-ischemic etiology does not imply a different prognosis nor, with exception of antiarrhythmic strategies, treatment modality. Likewise, except for pancreatic-islet-transplant issues, the fundamental and adjunctive therapies for long standing, insulin-dependent T2D are the same, regardless the underlying reasons for T2D. Once advanced stages of T2D have been reached, different etiologies, i.e. genetically or environmentally caused T2D, appear of less importance to disease management and prognosis. Therefore, uniform disease mechanisms pertinent for disease progression in T2D need to be identified. As such a mechanism, neurohumoral stimulation comprising autonomic-nervous-system (ANS) dysfunction, i.e. sympathetic activation and vagal deactivation, as well as activation of hormones such as the renin-angiotensin-aldosterone system (RAAS) may be considered. The concept of neurohumoral stimulation was first applied to CHF, where plasma norepinephrine [1], atrial natriuretic peptide [2], B-type natriuretic peptide [3] and endothelin-1 [4] were found to be correlated with mortality or severity of disease. In T2D neurohumoral stimulation exists as well. There, ANS dysfunction [5] and the RAAS [6, 7] are critically involved. In addition, neurohumoral stimulation may involve adipose-tissue hormones given that obesity is one risk factor for T2D [8] as well as gastrointestinal neuropeptidergic inputs [9]. Hormone actions may affect insulin sensitivity, oxidative stress, inflammation and endothelial function (Table 1) as well as the ANS (Table 2).
Presentation of the hypothesis
We hypothesize that persistent sympathetic-nervous-system activation actually represents one cause of peripheral insulin resistance (IR) defined as partial blocking of insulin effects on glucose uptake emphasizing oxidative stress and inflammation as possible links between neurohumoral stimulation and IR.
Testing the hypothesis
First, T2D-relevant hormones and the ANS are reviewed. Second, consequences of neurohumoral stimulation such as oxidative stress and inflammation are highlighted with regard to IR.
Insulin
Beyond the hypoglycemic action, insulin activates the sympathetic nervous system, while withdrawing the vagal component of the ANS [10, 11]. Furthermore, insulin enhances endothelial nitric-oxide synthase (eNOS) [12] which determines microvascular tone and may neutralize reactive oxygen species (ROS). We hypothesize that ANS dysfunction in terms of sympathetic activation, from whatever source, deteriorates peripheral insulin sensitivity, thus leading to repetitive hyperinsulinemia and hyperglycemia. If this hypothesis holds true, resulting hyperinsulinemia may further activate the sympathetic component of the ANS. Moreover, as hallmarks of type-2 diabetes, continued hyperinsulinemia may lead to pancreatic beta-cell exhaustion [15], while repetitive hyperglycemia may increase oxidative stress [13, 14].
Adipokines
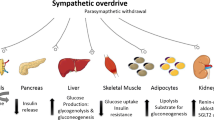
Obesity is a major risk factor for T2D [16]. Adipokines such as leptin, resistin and adiponectin seem to be involved in IR. As stated in Figure 1, hyperleptinemia occurring in obesity directly activates the sympathetic nervous system [17–19]. Leptin increases oxidative stress [20], however, it may further attenuate interleukin activation [21]. Both leptin and resistin serum levels have been found to be associated with IR [22]. Conversely, the duodenum-derived ghrelin [9] and adiponectin [23, 24] were demonstrated to correlate with insulin sensitivity. ANS effects of resistin, adiponectin or of ghrelin are currently unknown. However, on endothelial cells, resistin promotes the release of endothelin-1 [25], known to be sympatho-excitatory [26]. Besides the possible role of adipokines on the ANS, cellular and subcellular adipocyte signalling issues may yield further insight into the special role of abdominal obesity for T2D [27].
Catecholamines
Catecholamines deteriorate insulin sensitivity as shown by high plasma concentrations of catecholamines in pheochromocytoma patients. There, insulin sensitivity was restored by tumor-removal surgery [28]. On the molecular level β-adrenergic stimulation itself inhibits insulin signaling molecules such as insulin receptor substrates, which have been shown to be essential for insulin action [29–31]. Furthermore, expression of adiponectin is inhibited, whereas IR-inducing IL-6 is stimulated by β-adrenergic activation in fat cells [32, 33]. However, beta-adrenergic receptor blocking drugs have not shown any effect on insulin sensitivity [34]. Both metabolic counteractive effects of beta-adrenergic blockade on pancreas-islet function and possibly still-active sympathetic stimulation via autonomic nerve fibers [35] may explain the lack of effect of beta-adrenergic blockade on IR. However, third-generation, vasodilating beta-blocking agents may set off possible adverse metabolic effects and confer a benefit for individuals having IR [36] bearing in mind that actions of epinephrine and norepinephrine include pro-oxidative [37] and proinflammatory effects [38, 39].
Renin-Angiotensin-Aldosterone System
Recent studies demonstrate the beneficial nature of therapeutic angiotensin-2 blockade in T2D using either angiotensin-converting-enzyme (ACE) inhibitors or direct angiotensin-2, subtype-1 receptor (AT1) antagonists [7, 40, 41]. Several mechanisms may account for this. First, angiotensin 2 acts as a sympathetic-nervous-system activator [42] and as a deactivator of vagal function [43]. Secondly, the activation of the RAAS facilitates the generation of free ROS via a NADPH-oxidase mechanism [44], leading to oxidative injury [45] and IR. Furthermore, angiotensin 2 elicits a pro-inflammatory, monocytic response [46]. Conversely, the blockade of the RAAS elicits a reduction of oxidative stress [47], a decrease of CRP as a marker of inflammation [48], a restoration of endothelial function [49], and an increase of adiponectin concentration [50], indicating an adipocyte-mediated amelioration of insulin sensitivity [23].
Aldosterone may independently contribute to adverse effects, given the observation, that aldosterone release may "escape" an ACE-inhibition or AT1 blockade [51]. Pharmacological or surgical correction of hyperaldosteronism has been shown to improve insulin sensitivity [52]. Moreover, an elevated plasma aldosterone is associated with pro-inflammatory and pro-oxidative effects [53] and may confer endothelial dysfunction [54]. Indirect evidence suggests aldosterone to be associated with an attenuated vagal [55] and cardiac sympathetic function [56]. However, especially for aldosterone effects on the sympathetic nervous system, there is no conclusive evidence available, yet.
Endothelin-1
Endothelin-1 is a most potent, mainly endothelially derived vasoconstrictor. Endothelin-1 concentrations were found to be increased in T2D [57]. Endothelin-receptor-A blockade improves endothelial dysfunction in T2D and obesity [58]. Moreover, chronic endothelin-1 application is associated with elevated oxidative stress [59, 60], inflammatory reaction within the arterial wall [61] and IR [62]. The principle of endothelin-1 blockade may, therefore, have beneficial effects in patients with T2D. Those effects may include an attenuation of prevalent sympatho-excitation as shown in the CHF condition [26].
Autonomic-nervous-system dysfunction as a cause of insulin resistance
Paradoxically, ANS dysfunction in terms of an activation of the sympathetic nervous system either increases or blunts insulin sensitivity. On the one hand, sympathetic activation may increase energy expenditure [63] and mediate lipolysis [64], thereby possibly reducing overweight as beta adrenergic blockade implies [65]. Thus, sympathetic activation may primarily serve as a compensatory mechanism. On the other hand, too much sympathetic activation may lead to IR: caffeine, eliciting sympathetic activation, has been shown to diminish peripheral-tissue insulin sensitivity [66]. Pheochromocytoma patients clearly have IR due to the high plasma concentrations of norepinephrine [28], and non-diabetic patients with sympatho-excitation due to spinal-chord injury were shown to have IR [67]. Moreover, heart-rate recovery following exercise-capacity tests as a measure of vagal function and predictor of cardiovascular mortality [68], correlates with insulin sensitivity [69]. Thus, once sympatho-activation or vagal deactivation is persistent, deleterious consequences such as IR may occur. Therefore, we hypothesize that sympatho-excitation, i.e. persistent sympathetic activation, may cause IR either directly via signalling effects within target cells or, indirectly, via oxidative-stress induced inflammatory responses. The attenuation of sympatho-excitation by centrally acting antiadrenergic drugs such as moxonidin slightly reduced IR [70] as a further indication of the validity of our hypothesis.
Mechanistically, a reduced nitric-oxide (NO) availability may be involved in the evolution of sympatho-excitation given the fact that certain eNOS polymorphisms are concomitant with a higher T2D and IR susceptibility [71]. Besides cardiovascular NO effects, central-nervous system NO may exert sympatho-inhibitory effects [72, 73].
Furthermore, life-style modifications such as exercise may restore ANS function in T2D or in precursor states via restoration of cardiovascular reflexes [74]. External factors, such as ethanol and tobacco consumption may further contribute to oxidative stress [75, 76] or sympathetic-nervous-system activation [77, 78].
The individual players of the overall concept of neurohumoral stimulation must still be characterized. As Figure 1 shows, the known T2D risk factors may ultimately affect the sympathetic nervous system, the RAAS and oxidative-stress milieu, once antioxidant pathways are saturated.
Oxidative stress and insulin resistance
Oxidative stress is a candidate mechanism connecting neurohumoral stimulation with IR [79], involving either an activated, vascular-smooth-muscle NADPH oxidase [44] or uncoupled nitric-oxide synthase. Those sources of "internal" oxidative-stress may be therapeutic targets. Other sources of internal oxidative stress, such as cytochrome-c leakage of superoxide anion, may be involved in (as well being the reason for) oxidative-stress sequelae such as carbonylation of proteins as seen in T2D [80]. Besides subcellular effects on insulin signalling [81], oxidative stress may lead to an activation of cytokines [82, 83] as well as to an activation of the sympathetic nervous system [84], possibly via desensitized afferent fibers of the sympatho-inhibitory baroreflex [85]. Thus, once RAAS-mediated oxidative stress is not compensated, another vicious cycle begins, aggravating IR towards T2D. According to Figure 2, it appears to be important to intervene in the possible vicious cycles involving the activation of the RAAS and the sympathetic nervous system. As both ACE inhibitors and AT1 antagonists interfere with the RAAS, hydroxy-methyl-glutaryl-CoA-reductase inhibitors or statins may have the potential to attenuate sympatho-excitation [73, 86] possibly by preventing NADPH oxidase activation as a source of endogenous oxidative stress [14, 87]. In both type-1 and type-2-diabetes mellitus, the latter drug clearly improved outcomes [88], however, peroral antioxidants did not [89]. One may argue that oxidative stress associated with T2D, atherosclerosis and hypertension is therapeutically accessible solely by drugs affecting internal sources of oxidative stress such as statins [88] or AT1 blockade [41]. Besides inflammation, vasodilatory or endothelial dysfunction may be another consequence of enhanced oxidative stress by ROS scavenging endothelial-cell derived nitric oxide [90].
Suggested therapeutic interventions with regard to the framework of T2D risk factors and neurohumoral factors given in Figure 1. Solid lines across empty arrows signify blocked pathways; dotted lines across solid arrows represent attenuated pathways.
Inflammation and insulin resistance
Inflammatory responses are likely to be due to oxidative-stress mediated alterations of cytokine expression including interleukin-6 [83]. Interleukin-6, in turn, elicits a CRP release from the liver [91]. The principle of ROS-mediated inflammation has been demonstrated using ultraviolet light [92, 93]. Although more research is needed, this suggested relationship may underly the inflammatory responses seen in atherosclerosis, obesity, and T2D. Prevalent inflammation may at least partially explain the occurrence of IR amid neurohumoral stimulation given the known roles of cytokines and of acute-phase reactants in IR [94, 95]. In fact, inflammation has been shown to be one risk factor for T2D [96]. As yet another proposed vicious cycle, repetitive hyperglycemia due to IR may further aggravate the oxidative stress [14] as well as the cytokine-mediated inflammatory response (Figure 1).
Implications of the hypothesis
The summarized evidence suggests that neuroendocrine mechanisms participate in T2D evolution. Neurohumoral stimulation is likely to be an important cause for the oxidative-stress milieu, the state of inflammation, and insulin resistance. Therefore, we propose the hypothesis that ANS dysfunction, most likely sympathetic-nervous-system activation, represents one cause of IR likely to be mediated by oxidative-stress induced inflammation. For both T2D treatment and prevention, this hypothesis may serve as a rationale for both pharmaceutical and non-pharmaceutical interventions to restore ANS tone and insulin sensitivity. Treatments, that are likely to affect ANS tone and insulin sensitivity may include RAAS interference, statin therapy, endothelin antagonism, or weight-control programs through exercise and/or hypocaloric diet (Figure 2). Because IR is difficult to measure, a practical approach may involve non-diabetic individuals having 'metabolic syndrome' [97]. Metabolic syndrome implies a 24.5-fold elevated risk for incidence of T2D [98] probably due to an underlying peripheral IR. Disturbed autonomic activity in terms of sympathetic activation and vagal deactivation may be a candidate mechanism for both the conversion processes of obesity to metabolic syndrome and of metabolic syndrome to T2D. Overall, clinical studies in T2D patients as well as in patients at risk for the development of T2D should determine ANS surrogates, such as muscle sympathetic nerve activity or heart rate variability, to further elucidate the role of ANS dysfunction in T2D evolution.
References
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T: Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984, 311: 819-823.
Isnard R, Pousset F, Trochu J, Chafirovskaia O, Carayon A, Golmard J, Lechat P, Thomas D, Bouhour J, Komajda M: Prognostic value of neurohormonal activation and cardiopulmonary exercise testing in patients with chronic heart failure. Am J Cardiol. 2000, 86: 417-421. 10.1016/S0002-9149(00)00957-7.
Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R: B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002, 105: 2392-2397. 10.1161/01.CIR.0000016642.15031.34.
Rodeheffer RJ, Lerman A, Heublein DM, Burnett JC: Increased plasma concentrations of endothelin in congestive heart failure in humans. Mayo Clin Proc. 1992, 67: 719-724.
Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D: Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation. 2003, 107: 2190-2195. 10.1161/01.CIR.0000066324.74807.95.
Lindholm LH, Ibsen H, Borch J, Olsen MH, Wachtell K, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S: Risk of new-onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2002, 20: 1879-1886. 10.1097/00004872-200209000-00035.
Brenner BM, Cooper ME, de Z, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001, 345: 861-869. 10.1056/NEJMoa011161.
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA. 2003, 289: 76-79. 10.1001/jama.289.1.76.
Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O: Low Plasma Ghrelin Is Associated With Insulin Resistance, Hypertension, and the Prevalence of Type 2 Diabetes. Diabetes. 2003, 52: 2546-2553.
Muntzel MS, Anderson EA, Johnson AK, Mark AL: Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens. 1995, 17: 39-50.
Van De Borne P, Hausberg M, Hoffman RP, Mark AL, Anderson EA: Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am J Physiol. 1999, 276: R178-R183.
Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL: Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000, 101: 676-681.
Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ: Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002, 25: 537-541.
Christ M, Bauersachs J, Liebetrau C, Heck M, Gunther A, Wehling M: Glucose increases endothelial-dependent superoxide formation in coronary arteries by NAD(P)H oxidase activation: attenuation by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor atorvastatin. Diabetes. 2002, 51: 2648-2652.
Katsuragi I, Okeda T, Yoshimatsu H, Utsunomiya N, Ina K, Sakata T: Transplantation of normal islets into the portal vein of Otsuka Long Evans Tokushima Fatty rats prevents diabetic progression. Exp Biol Med. 2001, 226: 681-685.
Burke JP, Williams K, Narayan KM, Leibson C, Haffner SM, Stern MP: A population perspective on diabetes prevention: whom should we target for preventing weight gain?. Diabetes Care. 2003, 26: 1999-2004.
Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI: Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997, 100: 270-278.
Gudbjornsdottir S, Lonnroth P, Sverrisdottir YB, Wallin BG, Elam M: Sympathetic Nerve Activity and Insulin in Obese Normotensive and Hypertensive Men. Hypertension. 1996, 27: 276-280.
Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M: Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003, 41: 1072-1079. 10.1161/01.HYP.0000066289.17754.49.
Beltowski J, Wojcicka G, Jamroz A: Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: the possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis. 2003, 170: 21-29. 10.1016/S0021-9150(03)00236-3.
Xiao E, Xia-Zhang L, Vulliemoz NR, Ferin M, Wardlaw SL: Leptin Modulates Inflammatory Cytokine and Neuroendocrine Responses to Endotoxin in the Primate. Endocrinology. 2003, 144: 4350-4353.
Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ: Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003, 149: 331-335.
Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C: Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002, 51: 1884-1888.
Pellme F, Smith U, Funahashi T, Matsuzawa Y, Brekke H, Wiklund O, Taskinen MR, Jansson PA: Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of type 2 diabetic patients. Diabetes. 2003, 52: 1182-1186.
Verma S, Li SH, Wang CH, Fedak PWM, Li RK, Weisel RD, Mickle DAG: Resistin Promotes Endothelial Cell Activation: Further Evidence of Adipokine-Endothelial Interaction. Circulation. 2003, 108: 736-740. 10.1161/01.CIR.0000084503.91330.49.
Liu JL, Pliquett RU, Brewer E, Cornish KG, Shen YT, Zucker IH: Chronic endothelin-1 blockade reduces sympathetic nerve activity in rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol. 2001, 280: R1906-R1913.
Abu-Elheiga L, Oh W, Kordari P, Wakil SJ: Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. PNAS. 2003, 100: 10207-10212. 10.1073/pnas.1733877100.
Wiesner TD, Bluher M, Windgassen M, Paschke R: Improvement of Insulin Sensitivity after Adrenalectomy in Patients with Pheochromocytoma. J Clin Endocrinol Metab. 2003, 88: 3632-3636. 10.1210/jc.2003-030000.
Fasshauer M, Klein J, Kriauciunas KM, Ueki K, Benito M, Kahn CR: Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol Cell Biol. 2001, 21: 319-329. 10.1128/MCB.21.1.319-329.2001.
Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, Kahn CR: Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem. 2000, 275: 25494-25501. 10.1074/jbc.M004046200.
Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR: beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999, 274: 34795-34802. 10.1074/jbc.274.49.34795.
Fasshauer M, Klein J, Lossner U, Paschke R: Interleukin (IL)-6 mRNA Expression is Stimulated by Insulin, Isoproterenol, Tumour Necrosis Factor Alpha, Growth Hormone, and IL-6 in 3T3-L1 Adipocytes. Horm Metab Res. 2003, 35: 147-152. 10.1055/s-2003-39075.
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R: Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001, 507: 142-146. 10.1016/S0014-5793(01)02960-X.
Kusunoki M, Oshida Y, Iguchi A, Iida T, Suga T, Funado T, Sato Y, Kato K, Sakamoto N: Influence of sympatho-adrenal system on insulin sensitivity using the euglycemic clamp technique. Diabetes Res Clin Pract. 1992, 17: 125-131. 10.1016/0168-8227(92)90157-M.
Turtzo LC, Marx R, Lane MD: Cross-talk between sympathetic neurons and adipocytes in coculture. PNAS. 2001, 98: 12385-12390. 10.1073/pnas.231478898.
Jacob S, Balletshofer B, Henriksen EJ, Volk A, Mehnert B, Loblein K, Haring HU, Rett K: Beta-blocking agents in patients with insulin resistance: effects of vasodilating beta-blockers. Blood Press. 1999, 8: 261-268. 10.1080/080370599439463.
Aizawa T, Ishizaka N, Usui S, Ohashi N, Ohno M, Nagai R: Angiotensin II and catecholamines increase plasma levels of 8-epi-prostaglandin F(2alpha) with different pressor dependencies in rats. Hypertension. 2002, 39: 149-154. 10.1161/hy1201.097301.
Schade R, Gohler K, Burger W, Hirschelmann R: Modulation of rat C-reactive protein serum level by dexamethasone and adrenaline – comparison with the response of alpha 2-acute phase globulin. Agents Actions. 1987, 22: 280-287.
Takahashi T, Anzai T, Yoshikawa T, Maekawa Y, Asakura Y, Satoh T, Mitamura H, Ogawa S: Serum C-reactive protein elevation in left ventricular remodeling after acute myocardial infarction – role of neurohormones and cytokines. In J Cardiol. 2003, 88: 257-265. 10.1016/S0167-5273(02)00416-3.
Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K: Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002, 359: 1004-1010. 10.1016/S0140-6736(02)08090-X.
Heart Outcomes Prevention Evaluation Study Investigators: Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000, 355: 253-259. 10.1016/S0140-6736(99)12323-7.
Esler M: Differentiation in the effects of the angiotensin II receptor blocker class on autonomic function. J Hypertens. 2002, 20 (Suppl 5): S13-S19.
Le M, Mimassi N, Lancien F, Mabin D, Boucher JM, Blanc JJ: Heart rate variability, a target for the effects of angiotensin II in the brain of the trout Oncorhynchus mykiss. Brain Res. 2002, 947: 34-40. 10.1016/S0006-8993(02)02903-7.
Sowers JR: Hypertension, Angiotensin II, and Oxidative Stress. N Engl J Med. 2002, 346: 1999-2001. 10.1056/NEJMe020054.
Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M: Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002, 40: 872-879. 10.1161/01.HYP.0000040262.48405.A8.
Kranzhofer R, Browatzki M, Schmidt J, Kubler W: Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun. 1999, 257: 826-828. 10.1006/bbrc.1999.0543.
Koh KK, Ahn JY, Han SH, Kim DS, Jin DK, Kim HS, Shin MS, Ahn TH, Choi IS, Shin EK: Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003, 42: 905-910. 10.1016/S0735-1097(03)00846-5.
Takeda T, Hoshida S, Nishino M, Tanouchi J, Otsu K, Hori M: Relationship between effects of statins, aspirin and angiotensin II modulators on high-sensitive C-reactive protein levels. Atherosclerosis. 2003, 169: 155-158. 10.1016/S0021-9150(03)00158-8.
Hornig B, Kohler C, Schlink D, Tatge H, Drexler H: AT1-Receptor Antagonism Improves Endothelial Function in Coronary Artery Disease by a Bradykinin/B2-Receptor-Dependent Mechanism. Hypertension. 2003, 41: 1092-1095. 10.1161/01.HYP.0000064942.77814.26.
Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K: Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003, 42: 76-81. 10.1161/01.HYP.0000078490.59735.6E.
Sato A, Hayashi K, Naruse M, Saruta T: Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003, 41: 64-68. 10.1161/01.HYP.0000044937.95080.E9.
Haluzik M, Sindelka G, Widimsky J, Prazny M, Zelinka T, Skrha J: Serum leptin levels in patients with primary hyperaldosteronism before and after treatment: relationships to insulin sensitivity. J Hum Hypertens. 2002, 16: 41-45. 10.1038/sj.jhh.1001292.
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT: Aldosterone-Induced Inflammation in the Rat Heart : Role of Oxidative Stress. Am J Pathol. 2002, 161: 1773-1781.
Kurbanov RD, Eliseeva MR, Tursunov RR, Kurbanova DR, Zakirova FA: [Humoral markers of endothelial dysfunction in essential hypertension]. Kardiologiia. 2003, 43: 61-64.
Korkmaz ME, Muderrisoglu H, Ulucam M, Ozin B: Effects of spironolactone on heart rate variability and left ventricular systolic function in severe ischemic heart failure. Am J Cardiol. 2000, 86: 649-653. 10.1016/S0002-9149(00)01046-8.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M: Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003, 41: 574-581. 10.1016/S0735-1097(02)02855-3.
Cardillo C, Campia U, Bryant MB, Panza JA: Increased Activity of Endogenous Endothelin in Patients With Type II Diabetes Mellitus. Circulation. 2002, 106: 1783-10.1161/01.CIR.0000032260.01569.64.
Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD: Endothelin Contributes to Basal Vascular Tone and Endothelial Dysfunction in Human Obesity and Type 2 Diabetes. Diabetes. 2002, 51: 3517-3523.
Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H: Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000, 269: 713-717. 10.1006/bbrc.2000.2354.
Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF: Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003, 107: 1053-1058. 10.1161/01.CIR.0000051459.74466.46.
Browatzki M, Schmidt J, Kubler W, Kranzhofer R: Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res Cardiol. 2000, 95: 98-105. 10.1007/s003950050170.
Wilkes JJ, Hevener A, Olefsky J: Chronic endothelin-1 treatment leads to insulin resistance in vivo. Diabetes. 2003, 52: 1904-1909.
Hawkins MF, Avery DD: Effects of centrally-administered bombesin and adrenalectomy on behavioral thermoregulation and locomotor activity. Neuropharmacology. 1983, 22: 1249-1255. 10.1016/0028-3908(83)90197-1.
Hucking K, Hamilton W, Ellmerer M, Bergman RN: Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003, 111: 257-264. 10.1172/JCI200314466.
Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC: Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension. 2001, 37: 250-254.
Keijzers GB, De Galan BE, Tack CJ, Smits P: Caffeine Can Decrease Insulin Sensitivity in Humans. Diabetes Care. 2002, 25: 364.
Karlsson AK: Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999, 37: 494-500. 10.1038/sj.sc.3100844.
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS: Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality. N Engl J Med. 1999, 341: 1351-1357. 10.1056/NEJM199910283411804.
Lind L, Andren B: Heart rate recovery after exercise is related to the insulin resistance syndrome and heart rate variability in elderly men. Am Heart J. 2002, 144: 666-672. 10.1016/S0002-8703(02)00138-2.
Haenni A, Lithell H: Moxonidine improves insulin sensitivity in insulin-resistant hypertensives. J Hypertens. 1999, 17 (Suppl 3): S29-S35.
Monti LD, Barlassina C, Citterio L, Galluccio E, Berzuini C, Setola E, Valsecchi G, Lucotti P, Pozza G, Bernardinelli L: Endothelial Nitric Oxide Synthase Polymorphisms Are Associated With Type 2 Diabetes and the Insulin Resistance Syndrome. Diabetes. 2003, 52: 1270.
Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A: Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens. 2003, 21: 379-386. 10.1097/00004872-200302000-00030.
Pliquett RU, Cornish KG, Peuler JD, Zucker IH: Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003, 107: 2493-2498. 10.1161/01.CIR.0000065606.63163.B9.
De A, Schaan BD, Maeda CY, Dall A, Wichi RB, Irigoyen MC: Cardiovascular control in experimental diabetes. Braz J Med Biol Res. 2002, 35: 1091-1100.
Kay HH, Grindle KM, Magness RR: Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol. 2000, 182: 682-688.
Zhang J, Liu Y, Shi J, Larson DF, Watson RR: Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med (Maywood). 2002, 227: 823-829.
Hausberg M, Mark AL, Winniford MD, Brown RE, Somers VK: Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation. 1997, 96: 282-287.
Bennett AJ, Sponberg AC, Graham T, Suomi SJ, Higley JD, DePetrillo PB: Initial ethanol exposure results in decreased heart rate variability in ethanol-naive rhesus monkeys. Eur J Pharmacol. 2001, 433: 169-172. 10.1016/S0014-2999(01)01445-5.
Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Nakatani K, Yano Y, Adachi Y: Increased Oxidative Stress Is Associated With Serum Levels of Triglyceride, Insulin Resistance, and Hyperinsulinemia in Japanese Metabolically Obese, Normal-Weight Men. Diabetes Care. 2004, 27: 631-632.
Dalle D, Giustarini D, Colombo R, Rossi R, Milzani A: Protein carbonylation in human diseases. Trends Mol Med. 2003, 9: 169-176. 10.1016/S1471-4914(03)00031-5.
Tirosh A, Rudich A, Potashnik R, Bashan N: Oxidative stress impairs insulin but not platelet-derived growth factor signalling in 3T3-L1 adipocytes. Biochem J. 2001, 355: 757-763.
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D: Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002, 106: 2067-2072. 10.1161/01.CIR.0000034509.14906.AE.
Frossi B, De C, Daniel KC, Rivera J, Pucillo C: Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003, 33: 2168-2177. 10.1002/eji.200323995.
Girouard H, Chulak C, LeJossec M, Lamontagne D, De Champlain J: Chronic antioxidant treatment improves sympathetic functions and beta-adrenergic pathway in the spontaneously hypertensive rats. J Hypertens. 2003, 21: 179-188. 10.1097/00004872-200301000-00028.
Nightingale AK, Blackman DJ, Field R, Glover NJ, Pegge N, Mumford C, Schmitt M, Ellis GR, Morris T, Frenneaux MP: Role of nitric oxide and oxidative stress in baroreceptor dysfunction in patients with chronic heart failure. Clin Sci (Lond). 2003, 104: 529-535. 10.1042/CS20020334.
Pliquett RU, Cornish KG, Zucker IH: Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003, 95: 700-704.
Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U: Oxygen Free Radical Release in Human Failing Myocardium Is Associated With Increased Activity of Rac1-GTPase and Represents a Target for Statin Treatment. Circulation. 2003, 108: 1567-1574. 10.1161/01.CIR.0000091084.46500.BB.
Collins R, Armitage J, Parish S, Sleigh P, Peto R: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003, 361: 2005-2016. 10.1016/S0140-6736(03)12475-0.
Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002, 360: 23-33. 10.1016/S0140-6736(02)11807-1.
Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R: Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002, 39: 135-141. 10.1161/hy0102.100540.
Yasunari K, Maeda K, Nakamura M, Yoshikawa J: Oxidative stress in leukocytes is a possible link between blood pressure, blood glucose, and C-reacting protein. Hypertension. 2002, 39: 777-780. 10.1161/hy0302.104670.
Urbanski A, Schwarz T, Neuner P, Krutmann J, Kirnbauer R, Kock A, Luger TA: Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990, 94: 808-811.
Hruza LL, Pentland AP: Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993, 100: 35S-41S.
Festa A, Hanley AJ, Tracy RP, Agostino R, Haffner SM: Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003, 108: 1822-1830. 10.1161/01.CIR.0000091339.70120.53.
Festa A, Agostino R, Tracy RP, Haffner SM: Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002, 51: 1131-1137.
Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N: Elevated C-Reactive Protein Is a Risk Factor for the Development of Type 2 Diabetes in Japanese Americans. Diabetes Care. 2003, 26: 2754-2757.
Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA: Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002, 156: 1070-1077. 10.1093/aje/kwf145.
Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM: Metabolic Syndrome With and Without C-Reactive Protein as a Predictor of Coronary Heart Disease and Diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003, 108: 414-419. 10.1161/01.CIR.0000080897.52664.94.
Acknowledgements
The authors wish to thank Dr. M. Mann, University of Nebraska Medical Center, for his comments and help in the editing process.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
RUP constructed the hypothesis and drafted the manuscript. MF and MB added insight into the sections concerning adipokines and catecholamines. RP participated in finalizing the manuscript. All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pliquett, R., Fasshauer, M., Blüher, M. et al. Neurohumoral stimulation in type-2-diabetes as an emerging disease concept. Cardiovasc Diabetol 3, 4 (2004). https://doi.org/10.1186/1475-2840-3-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-3-4