Abstract
Our aim is to summarize and discuss the recent literature linking diabetes mellitus with heart failure, and to address the issue of the optimal treatment for diabetic patients with heart failure.
The studies linking diabetes mellitus (DM) with heart failure (HF)
The prevalence of diabetes mellitus in heart failure populations is close to 20% compared with 4 to 6% in control populations. Epidemiological studies have demonstrated an increased risk of heart failure in diabetics; moreover, in diabetic populations, poor glycemic control has been associated with an increased risk of heart failure. Various mechanisms may link diabetes mellitus to heart failure: firstly, associated comorbidities such as hypertension may play a role; secondly, diabetes accelerates the development of coronary atherosclerosis; thirdly, experimental and clinical studies support the existence of a specific diabetic cardiomyopathy related to microangiopathy, metabolic factors or myocardial fibrosis. Subgroup analyses of randomized trials demonstrate that diabetes is also an important prognostic factor in heart failure. In addition, it has been suggested that the deleterious impact of diabetes may be especially marked in patients with ischemic cardiomyopathy.
Treatment of heart failure in diabetic patients
The knowledge of the diabetic status may help to define the optimal therapeutic strategy for heart failure patients. Cornerstone treatments such as ACE inhibitors or beta-blockers appear to be uniformly beneficial in diabetic and non diabetic populations. However, in ischemic cardiomyopathy, the choice of the revascularization technique may differ according to diabetic status. Finally, clinical studies are needed to determine whether improved metabolic control might favorably influence the outcome of diabetic heart failure patients.
Similar content being viewed by others
Background
Heart failure (HF) is a major and growing public health issue. It is estimated that approximately 4 to 5 million Americans have HF, and that an additional 400,000 patients are diagnosed with HF each year [1]. HF prevalence is expected to reach 10 million cases in the U.S. by the year 2007 [2].
In spite of significant advances in management and treatment, the mortality of patients with HF remains high. In the CIBIS II (Cardiac Insufficiency Bisoprolol Study II) trial, after a median follow-up of 15 months, the all cause mortality was 11.8% in the group of patients receiving the beta-blocker bisoprolol [3]. In the ATLAS (Assessment of Treatment with Lisinopril And Survival) trial, after a median follow-up of 46 months, the all cause mortality was 42% in the group of patients randomized to high dose of the angiotensin converting enzyme (ACE) inhibitor lisinopril [4]. In unselected populations, the outcome is even worse. Data from the Medicare population demonstrated a 6-year mortality rate in HF patients of 84% in men and 77% in women [5]. In the EPICAL (Epidémiologie de l'Insuffisance Cardiaque Avancée en Lorraine) observational study, the all cause one-year mortality was 35.4% [6].
HF is also a major cause of morbidity; chronic HF results in almost 1 million hospitalizations each year in the U.S. [7]. This has a major impact on health care expenditure. In 1991, the total inpatient and outpatient costs for HF were estimated to be $38 billion (5.4% of the health care budget that year) [8]. As the population ages and the number of patients with HF increases, the economic burden of HF will inevitably increase [9].
Over recent years, the prevalence of diabetes mellitus (DM), in particular type II diabetes, has increased significantly. The prevalence of DM in adults worldwide was estimated to be 4% in 1995 and is projected to rise to 5.4% by the year 2025 [10]. In developed countries, the prevalence of DM is higher in the elderly (over 65 years) population [11] (Figure 1). DM is a well known and important risk factor for cardiac disease [12–15].
Prevalence of diabetes mellitus in an unselected population. This figure shows the prevalence of diabetes mellitus stratified by sex and age in the Framingham cohort. A higher proportion of diabetics is observed in subjects aged over 65 years. Adapted from reference [11].
While the most common cardiac manifestation in diabetic patients is coronary artery disease, DM also appears to be strongly linked to HF. Approximately 15 to 25% of patients with HF are diabetics [6, 16–18] and it has been suggested that DM may play an important role in the pathogenesis, prognosis, and response to treatment of HF [19]. In addition, advanced HF is related to marked insulin resistance [20]. The aim of this paper will be to summarize and discuss the available literature linking DM with HF, and to address the issue of the optimal treatment for diabetic patients with HF.
The studies linking DM with HF
The epidemiological evidence linking DM with HF
As shown by subgroup analyses of randomized studies, a significant proportion of patients with HF are diabetics (Figure 2). In the SOLVD (Studies of Left Ventricular Dysfunction) clinical trials, 15% of patients were diabetic in the Prevention arm and 26% in the Treatment arm [17]. In the V-HeFT II (Vasodilator Heart Failure Trial II), the proportion of patients with DM was 20% [16]. More recently, Ryden et al reported the results of the ATLAS study in patients with and without DM: of the 3164 patients included in the study, 611 (19%) were taking hypoglycemic agents (oral or insulin) at baseline and were considered as having clinical DM [18]. Information on the prevalence of DM in HF populations can also be obtained from registries; the unselected nature of the patients consecutively included in registries may provide a better estimate of the true rate of DM in patients with HF. The SOLVD Registry was conducted in conjunction with the Prevention and the Treatment trials and enrolled a large cohort of patients with an ejection fraction <45% to determine the baseline characteristics of a population with left ventricular dysfunction. A total of 6076 patients with left ventricular dysfunction were included in the SOLVD Registry; among these, 1425 (23%) were classified as diabetics by the investigators [17]. In the EPICAL study [6], a registry of consecutive patients hospitalized for advanced chronic HF due to left ventricular systolic dysfunction (ejection fraction <30%), 26% of patients had an history of type I or type II DM. Overall, the rate of DM in HF populations is thus close to 20%. This rate is much higher than the 4 to 6% prevalence of DM observed in age-matched control populations [10, 11] (Figure 1).
The first demonstration of an increased risk of HF in patients with DM was reported by Kannel and McGee [11] based on data obtained from 20 years follow-up of the Framingham cohort. The incidence of HF according to sex and diabetic status is shown in Figure 3A; an increased risk of HF was observed in patients with DM. Compared with non-diabetic males and females, the age-adjusted relative risks of HF for diabetic males and females were 2.20 and 5.37, respectively [11]. In a study by Tenenbaum et al in patients with ischemic heart disease, the incidence of HF at 6 to 9-year follow-up was 35.7% in non diabetic patients, 39% in patients with impaired fasting glucose and 45.7% in diabetic patients [21]. Other studies have demonstrated that the incidence of HF in diabetic patients is significantly correlated with HbA1c levels. This was primarily shown in the UK Prospective Diabetes Study (UKPDS) [22] (Figure 4A). These results were confirmed in a large population-based sample of 48,858 diabetic patients [23]; after adjustment for age and sex, each 1% increase in HbA1c was associated with a 12% increased risk of hospitalization for HF and/or death. These data demonstrating a strong association between HbA1c levels and HF in diabetic populations should be interpretated with caution; although poor glycemic control may be an independent risk factor for developing HF in diabetic populations, it is also conceivable that these data simply suggest a longer duration of DM, which is difficult to control, and therefore the development of HF may be more closely related to the duration of DM than to glycemic control.
Finally, although our aim was to review studies analyzing the risk of HF as a function of diabetic status, it must also be acknowledged that HF may predict future DM development; this has been demonstrated in an elderly population by Amato et al [24].
The mechanisms of HF in diabetic patients
DM may be causally related to HF development by at least 3 mechanisms: due to associated comorbidities, by favoring the development of coronary atherosclerosis, or through a specific diabetic cardiomyopathy.
Associated comorbidities or risk factors may partly account for the increased risk of HF in diabetic patients. These cardiovascular risk factors such as dyslipidaemia, hypertension, hypercoagulability, obesity and inflammation are part of the insulin resistance syndrome and are, at least partly, regulated by nuclear peroxisome proliferator-activated receptors (PPARs); activation of PPAR-gamma improve insulin sensitivity and endothelial function, and lower inflammation and blood pressure [25]. In the Framingham cohort, diabetic men and women had higher blood pressures and were more obese than non-diabetics; diabetic women had, in addition, higher LDL-cholesterol values; HDL-cholesterol values were consistently lower in those with DM than in those without DM in both sexes [26]. The same observation has been reported in HF populations: In the SOLVD trials [17, 27], for example, diabetic patients were older and were more likely to have a history of hypertension than non-diabetic patients: in the treatment arm, 54% of diabetics had hypertension versus 38% of non-diabetics (p < 0.001); in the prevention arm, 53% of diabetics had hypertension versus 34% of non-diabetics (p < 0.001). Although this may in part explain the higher incidence of HF in diabetic patients, other mechanisms must also play a role. Indeed, in most of the studies discussed previously, diabetes or poor glycemic control remained significantly associated with HF after adjustment for important baseline clinical variables including age, sex, and hypertension [11, 22, 23].
The increased risk of atherosclerosis in diabetic patients may also contribute significantly to the increased risk of HF. Coronary artery disease is the underlying cause of HF in approximately two thirds of patients with left ventricular systolic dysfunction [28]. DM is associated with a markedly increased risk of coronary artery disease. In the Framingham study, the incidence of coronary artery disease was increased in diabetic subjects (Figure 3B). In UKPDS, the risk of myocardial infarction increased as a function of HbA1c levels [22] (Figure 4B). In the study by Haffner et al [29], the seven-year incidence rate of myocardial infarction in diabetic subjects without prior myocardial infarction at baseline was 20.2% versus only 3.5% in non-diabetic subjects without prior myocardial infarction at baseline (Figure 5). This increased risk of atherosclerosis in diabetic subjects has been attributed to diverse mechanisms such as endothelial dysfunction [30] or altered hemostatic factors (higher levels of fibrinogen [31], plasminogen activator-inhibitor-1 [32, 33] or VonWillebrand factor [34]), or altered platelet function [35–38]). Molecular mechanisms linking hyperglycemia and atherosclerosis have been recently reviewed by Aronson et al [39].
Incidence of MI in diabetic versus nondiabetic populations. The 7-year incidence of MI is much higher in diabetics than in nondiabetics. This holds true for patients with or without prior MI. The risk of MI in diabetics without prior MI is similar to that observed in nondiabetics with prior MI. Adapted from reference [29].
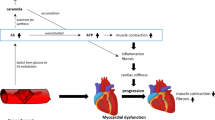
There are also data to suggest that DM may predispose to HF development through the existence of a specific diabetic cardiomyopathy [40]. The exact mechanism(s) by which DM may induce HF independent of epicardial coronary artery disease is (are) unknown but several hypotheses have been advanced; these include microangiopathy, metabolic factors, and fibrosis. Intramyocardial microangiopathy has also been observed in diabetic hearts [41–43]; combined with functional abnormalities related to endothelial dysfunction, diabetic microangiopathy may explain the reduced coronary blood flow reserve observed in diabetic patients [30, 44, 45]. Metabolic factors may also play a role in the development of myocardial dysfunction; hyperglycemia, impaired myocardial glucose uptake, and increased turnover of free fatty acids may all contribute to DM-related myocardial dysfunction (for review see [46–48]. Finally, experimental and clinical data also point to a potential role for myocardial fibrosis in diabetic cardiomyopathy; intramyocardial accumulation of collagen is a well-demonstrated consequence of DM [49, 50]; moreover, the deposition of advanced glycation end products (AGEs) may result in increased left ventricular stiffness and consequently to diastolic dysfunction [51–53]. In summary, various mechanisms may induce a specific diabetic cardiomyopathy. Whether this diabetic cardiomyopathy alone may cause HF is however unknown; another possibility is that these myocardial alterations related to DM may predispose to the development of HF in response to other insults such as coronary artery disease or hypertension (Figure 6). After an acute myocardial infarction, decreased compensatory responses of non-infarcted area have been described in diabetic patients [54–56]. Similarly, a synergistic effect may exist between DM and hypertension for the development of myocardial fibrosis [57].
Potential mechanisms linking diabetes mellitus to heart failure. Diabetes mellitus is associated with multiple physiopathological changes in the cardiovascular system. Among these, endothelial dysfunction and hemostatic disorders may at least in part account for the higher risk of coronary artery disease (CAD) while microangiopathy, myocardial fibrosis, and abnormal myocardial metabolism have been implicated in the pathogenesis of a specific diabetic cardiomyopathy. When it occurs in diabetic patients, heart failure (HF) is, in most cases, a consequence of CAD; other possible causes include the comorbidities frequently encountered in diabetic patients such as hypertension, or other causes of nonischemic cardiomyopathy. The existence of a diabetic cardiomyopathy may increase the risk of HF in response to these insults; however, whether diabetic cardiomyopathy alone may be responsible for HF remains unknown.
Determining diabetic status: an additional prognostic indicator in heart failure patients?
Risk stratification is an important step in the management of patients with HF; high risk patients may indeed benefit from more aggressive therapeutic strategies. Parameters such as New York Heart Association (NHYA) class, maximal VO2, left and right ventricular ejection fraction have been identified as powerful predictors of clinical outcome in HF patients [58–62].
The first suggestion that DM may be a predictor of poor clinical outcome in HF patients came in a report from Shindler et al [17]. A subgroup analysis of the SOLVD trials (combining the Prevention and the Treatment trials), showed that both all cause mortality and cardiovascular mortality at a mean follow-up of 3 years were significantly higher in diabetic patients than in non-diabetic patients (Figure 7). Multivariate analysis was used to assess the significance of DM as an independent predictor of outcome. After adjusting for important baseline variables such as age, sex, NYHA classification, or left ventricular ejection fraction, DM remained a significant predictor of clinical outcome in both the Prevention and the Treatment trials. More recently, Dries et al reanalyzed the SOLVD database to determine whether DM would have a different impact on clinical outcome in ischemic versus non-ischemic HF [27]. After adjustment for baseline variables, they found that DM was associated with an increased risk for all-cause mortality in patients with ischemic HF (RR 1.37, 95% CI 1.21 to 1.55), but not in patients with non-ischemic HF (RR 0.98, 95% CI 0.76 to 1.32) (Figure 8). Moreover, they suggested that the increased mortality in patients with ischemic HF compared with non-ischemic HF (reviewed in [63]) may be limited to the diabetic subgroup. If these findings are confirmed in independent studies, at least two explanations may account for the negative interaction between DM and the ischemic etiology of heart failure. Firstly, diabetic HF patients may have a higher risk of coronary plaque rupture and thrombosis [29, 64]; recurrent myocardial infarction is a major cause of death in patients with ischemic HF [65]; in addition, non fatal myocardial infarction may further deteriorate left ventricular function in patients with ischemic HF. Furthermore, the presence of various components of a specific diabetic cardiomyopathy such as impaired myocardial glucose uptake may be especially deleterious in patients with ischemic HF [66–69].
Diabetes mellitus as a predictor of clinical outcome in HF populations. All cause mortality and cardiovascular mortality are higher in diabetics than in nondiabetics. Adapted from the SOLVD trials [17].
Prognostic impact of diabetes mellitus according to HF etiology. Subgroup analysis of the SOLVD trials. The deleterious impact of diabetes mellitus is limited to the ischemic subgroup. Adapted from reference [27].
The links between DM and HF: the need for new studies
Most of the data on HF in diabetics summarized above have been obtained from post-hoc analysis of randomized studies or registries and as such should be interpreted with caution. In the SOLVD trial for example, the diagnosis of DM was solely based on self-reporting by the patient or on documentation in the patient's medical records and data on the duration of DM, severity of DM, and medications used to treat DM were not available. Similarly, in SOLVD, the definition of the etiology of HF (i.e., ischemic versus non ischemic) was based on the judgement of the investigators at the participating sites after reviewing all available information and did not routinely include cardiac catheterization or non-invasive testing.
New studies in HF populations with careful and prospective characterization of diabetic patients are needed; these studies may be designed either as ancillary studies of prospective randomized trials or as part of prospective registries on HF. The variables recorded should provide information on DM type and duration, and antidiabetic management (diet alone, oral hypoglycemic drugs, insulin). The presence/absence of signs of end-organ damage (retinopathy, neuropathy, nephropathy) would be a useful indicator of DM severity and duration and should also be recorded. Important biological variables related to the presence of DM or its complications (glycemia, HbA1c, serum creatinine, albuminuria, etc.) should also be prospectively determined. Finally, in view of the potential interactions between DM and CAD on HF risk and outcome, special attention should be given to prospective characterization of HF etiology (i.e., ischemic versus non ischemic).
Such studies would provide information on the characteristics of the diabetic cohort in HF populations and on the relationship between CAD and HF in diabetics. In addition, when coupled with clinical follow-up, these studies would allow propective confirmation of the hypothesis that DM has a deleterious impact on prognosis in HF patients and could determine whether biological markers such as HbA1c may serve as prognostic indicators in HF patients.
Treatment of HF in diabetic patients
Medical treatment
Post-hoc analyses of large randomized studies have shown that the beneficial effect of conventional HF treatment is maintained in the subgroup of diabetic patients. This has been conclusively demonstrated for the two classes of drugs, regarded as cornerstone treatments, namely ACE inhibitors and beta-blockers. In the SOLVD prevention and Treatment trials [70, 71], patients were randomized to either placebo or the ACE inhibitor enalapril; the efficacy was similar in diabetic and non-diabetic patients (Figure 7). There was no interaction between diabetic status and drug assignement with respect to the study endpoints [17]. In the ATLAS trial [4], patients were randomized to high or low doses lisinopril. The relative risk reduction in mortality for high-dose vs low-dose lisinopril was 14% for patients with diabetes mellitus and 6% for those without [18]; high-dose lisinopril was as effective in reducing hospitalizations for heart failure in diabetics as in non-diabetics (21% vs 24%) [18]. In ACE inhibitor-intolerant HF patients, the available literature supports the use of angiotensin II blockers [72]. In the CIBIS II trial, patients were randomized to placebo or the beta-blocker bisoprolol [3]; the efficacy was similar in diabetic and non-diabetic patients with respect to all mortality/morbidity endpoints [73]. For example, the relative risk (bisoprolol vs placebo) for mortality was 0.81 (95% CI 0.51–1.28) in diabetics and 0.66 (95% CI 0.54–0.81) in non-diabetics; the heterogeneity test for interaction was not statistically significant. Although these results were obtained from post-hoc analyses and as such have limitations from a methodological standpoint, the well-demonstrated benefits of ACE inhibitors and beta-blockers appears to be maintained in the diabetic subgroups. In addition, a similar relative risk reduction when applied to a high risk population such as diabetic HF patients will automatically translate into a major benefit in term of reduction in the absolute number of events.
In addition to ACE inhibitors and beta-blockers, patients with ischemic HF also benefit from secondary prevention with agents demonstrated to reduce atherosclerosis progression and to diminish the rate of acute coronary events. The use of antiplatelet agents was associated with an improvement in survival in patients with symptomatic or asymptomatic left ventricular dysfunction in the SOLVD study [74]. Statin therapy has been associated with an improved outcome in patients with coronary artery disease and left ventricular dysfunction [75]; moreover, in the 4S study, administration of simvastatin reduced the occurrence of HF [76]. Although no data are available concerning diabetic patients with ischemic HF, the demonstrated benefit of antiplatelet and statin therapy in diabetic patients with coronary artery disease [77–79] clearly supports a strategy of aggressive secondary prevention in diabetic patients with ischemic HF.
The need for new strategies/studies
Besides medical treatment for HF and the optimal use of secondary prevention strategies in cases with an ischemic origin, there are still important unanswered questions that will require further studies. For many diabetic patients with ischemic HF, the decision to revascularize and the choice of the revascularization technique are key issues. Moreover, the impact of DM treatment on HF outcome also needs to be considered.
Myocardial revascularization
Patients with ischemic cardiomyopathy represent an important subset of HF patients in whom myocardial revascularization may offer the potential for reduced symptoms and enhanced prognosis [80–84]. The optimal therapeutic strategy for coronary revascularization of diabetic patients is still a matter of debate [85–90]. Limited data are available regarding the relative merits of Coronary Artery Bypass Grafting (CABG) versus Percutaneous Transluminal Coronary Angioplasty (PTCA) in diabetic patients with ischemic HF: in a recent report of the Bypass Angioplasty Revascularization Investigation (BARI) study, the 7-year mortality was compared in patients randomized to CABG or PTCA according to the presence or absence of diabetes mellitus and left ventricular dysfunction at baseline [90]. In non-diabetic patients with left ventricular dysfunction the 7-year survival was similar in the CABG group and the PTCA group; on the other hand, in diabetic patients with left ventricular dysfunction CABG was associated with a better outcome than PTCA (Figure 9). Although these results support the choice of CABG as revascularization technique for diabetic patients with left ventricular dysfunction and multivessel coronary artery disease, it must be noted that patient selection and inclusion in the BARI study was performed >10 years ago [85]. Since then, new modalities of myocardial revascularization have been developed; the generalisation of the use of arterial grafts [85] and of coronary stent implantation [91, 92], and the advent of IIb/IIIa antagonists [93, 94] all have the potential to improve the outcome of diabetic HF patients undergoing myocardial revascularization. Similarly, the recent demonstration that drug eluting stents may significantly reduce the risk of restenosis could have a major impact in diabetic HF patients undergoing percutaneous coronary revascularization [95].
Survival in patients with multivessel CAD treated by CABG or PTCA according to presence/absence of diabetes mellitus and left ventricular dysfunction at baseline. Adapted from the BARI study [90]. In the diabetes mellitus (DM) subgroup, the outcome was better in the CABG group than in the PTCA group; this holds true in the presence or in the absence of left ventricular dysfunction.
Future studies will have to clarify the role of revascularization in diabetic patients with ischemic HF. It will be important to determine if revascularization in diabetics carries any advantage over medical therapy, a question that is currently under evaluation in the BARI 2D study (although not specifically in HF patients). If it is shown that revascularization improves prognosis, it would be appropriate to aggressively exclude an ischemic origin in diabetic HF patients.
Metabolic control
The impact of DM treatment in HF patients should also be considered. At the present time, it has not been determined whether improved metabolic control might favorably influence the outcome of diabetic HF patients and large clinical studies are urgently needed to provide an answer to this important question. The need for such studies is underlined by preliminary data suggesting that strict metabolic control may reverse to some extent the consequence of diabetic cardiomyopathy [96]. Such studies would also determine whether the preferred treatment for DM should be an insulin-sensitizing regimen or an insulin-providing regimen. Lifestyle interventions [97] (including dietary changes, increased physical activity and weigth loss) could also be specifically tested in diabetic HF patients. Finally, taking into account the possible interaction between HF etiology and the impact of metabolic control, prespecified subgroup analysis (non ischemic HF vs ischemic HF) would appear mandatory.
Conclusions
In summary, HF in diabetic patients is an important health problem. Approximately 20 to 25% of HF patients are diabetics. The review of the available literature suggests that the diabetic subgroup of HF patients deserves special consideration: at the present time, the natural history of HF in diabetic patients appears different with a higher mortality especially in the case of ischemic HF; moreover, although conventional HF treatments appear to be uniformly beneficial, in the case of ischemic HF the choice of the revascularization technique may differ according to diabetic status. Thus, an early and precise characterization of diabetic status should be encouraged not only in future clinical trials but also in everyday management of HF patients.
The present review underscores the need for new studies to help unravel the interplay between diabetes, atherosclerosis, and heart failure and to determine the specific role of currently available and novel therapies in the diabetic population.
Finally, a better understanding of the mechanisms leading to HF in diabetic patients may also help to design preventive strategies. At the present time, the well-documented beneficial effects of primary prevention of CAD in diabetics supports the preventive use of drugs such as statins [98] and ACE inhibitors [99]; other aspects such as for example careful blood pressure control [100] may also have a tremendous impact on the prevention of HF in this high risk population.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- AGEs:
-
Advanced Glycation End products
- ATLAS:
-
Assessment of Treatment with Lisinopril and Survival trial
- BARI:
-
Bypass Angioplasty Revascularization Investigation
- CABG:
-
Coronary Artery Bypass Grafting
- CIBIS II:
-
Cardiac Insufficiency Bisoprolol Study II
- DM:
-
Diabetes Mellitus
- EPICAL:
-
Epidémiologie de l'Insuffisance Cardiaque Avancée en Lorraine
- HF:
-
Heart failure
- NYHA:
-
New York Heart Association
- PTCA:
-
Percutaneous Transluminal Coronary Angioplasty
- SOLVD:
-
Studies of Left Ventricular Dysfunction
- UKPDS:
-
UK Prospective Diabetes Study
- V-HeFT II:
-
Vasodilator Heart Failure Trial II
References
American-Heart-Association: 1999 Heart and Stroke Statistical Update. In: Book 1999 Heart and Stroke Statistical Update (Editor ed.). 1998, City: American Heart Association
Rich MW: Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997, 45: 974-968.
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999, 353: 9-13.
Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Ryden L, Thygesen K, Uretsky BF: Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999, 100: 2312-2318.
Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF: Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med. 1999, 159: 505-510.
Zannad F, Braincon S, Juilliere Y, Mertes PM, Villemot JP, Alla F, Virion JM: Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l'Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol. 1999, 33: 734-742.
Levit KR, Lazenby HC, Cowan CA, Letsch SW: National health expenditures, 1990. Health Care Financ Rev. 1991, 13: 29-54.
O'Connell JB, Bristow MR: Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994, 13: S107-112.
O'Connell JB: The economic burden of heart failure. Clin Cardiol. 2000, 23: III6-10.
King H, Aubert RE, Herman WH: Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998, 21: 1414-1431.
Kannel WB, McGee DL: Diabetes and cardiovascular disease. The Framingham study. Jama. 1979, 241: 2035-2038.
Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL: The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984, 33: 271-276.
Krolewski AS, Warram JH, Rand LI, Kahn CR: Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med. 1987, 317: 1390-1398.
Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ: Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996, 16: 720-726.
Tuomilehto J, Borch-Johnsen K, Molarius A, Forsen T, Rastenyte D, Sarti C, Reunanen A: Incidence of cardiovascular disease in Type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia. 1998, 41: 784-790.
Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M: A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991, 325: 303-310.
Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC: Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996, 77: 1017-1020.
Ryden L, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA: Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000, 21: 1967-1978.
Solang L, Malmberg K, Ryden L: Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J. 1999, 20: 789-795.
Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ: Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997, 30: 527-532.
Tenenbaum A, Motro M, Fisman EZ, Leor J, Boyko V, Mandelzweig L, Behar S: Status of glucose metabolism in patients with heart failure secondary to coronary artery disease. Am J Cardiol. 2002, 90: 529-532.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000, 321: 405-412.
Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV: Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001, 103: 2668-2673.
Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, Varricchio M, Rengo F: Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab. 1997, 23: 213-218.
Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ: Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002, 62: 1463-1480.
Kannel WB, McGee DL: Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979, 2: 120-126.
Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ: Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001, 38: 421-428.
Gheorghiade M, Bonow RO: Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998, 97: 282-289.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998, 339: 229-334.
Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR: Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993, 42: 1017-25.
Ceriello A: Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993, 36: 1119-25.
Nordt TK, Sawa H, Fujii S, Sobel BE: Induction of plasminogen activator inhibitor type-1 (PAI-1) by proinsulin and insulin in vivo. Circulation. 1995, 91: 764-770.
Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H: Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998, 97: 2213-2221.
Zareba W, Pancio G, Moss AJ, Kalaria VG, Marder VJ, Weiss HJ, Watelet LF, Sparks CE: Increased level of von Willebrand factor is significantly and independently associated with diabetes in postinfarction patients. THROMBO Investigators. Thromb Haemost. 2001, 86: 791-799.
Knobler H, Savion N, Shenkman B, Kotev-Emeth S, Varon D: Shear-induced platelet adhesion and aggregation on subendothelium are increased in diabetic patients. Thromb Res. 1998, 90: 181-190.
Shechter M, Merz CN, Paul-Labrador MJ, Kaul S: Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol. 2000, 35: 300-307.
Jilma B, Fasching P, Ruthner C, Rumplmayr A, Ruzicka S, Kapiotis S, Wagner OF, Eichler HG: Elevated circulating P-selectin in insulin dependent diabetes mellitus. Thromb Haemost. 1996, 76: 328-332.
Tschoepe D, Roesen P, Kaufmann L, Schauseil S, Kehrel B, Ostermann H, Gries FA: Evidence for abnormal platelet glycoprotein expression in diabetes mellitus. Eur J Clin Invest. 1990, 20: 166-170.
Aronson D, Rayfield EJ: How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002, 1: 1.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A: New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972, 30: 595-602.
Factor SM, Okun EM, Minase T: Capillary microaneurysms in the human diabetic heart. N Engl J Med. 1980, 302: 384-388.
Gherasim L, Tasca C, Havriliuc C, Vasilescu C: A morphological quantitative study of small vessels in diabetic cardiomyopathy. Morphol Embryol (Bucur). 1985, 31: 191-195.
Yarom R, Zirkin H, Stammler G, Rose AG: Human coronary microvessels in diabetes and ischaemia. Morphometric study of autopsy material. J Pathol. 1992, 166: 265-270.
Nitenberg A, Paycha F, Ledoux S, Sachs R, Attali JR, Valensi P: Coronary artery responses to physiological stimuli are improved by deferoxamine but not by L-arginine in non-insulin-dependent diabetic patients with angiographically normal coronary arteries and no other risk factors. Circulation. 1998, 97: 736-743.
Nahser PJ, Brown RE, Oskarsson H, Winniford MD, Rossen JD: Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995, 91: 635-640.
Rodrigues B, McNeill JH: The diabetic heart: metabolic causes for the development of a cardiomyopathy. Cardiovasc Res. 1992, 26: 913-922.
Rodrigues B, Cam MC, McNeill JH: Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998, 180: 53-57.
Lopaschuk GD: Abnormal mechanical function in diabetes: relationship to altered myocardial carbohydrate/lipid metabolism. Coron Artery Dis. 1996, 7: 116-123.
Regan TJ, Wu CF, Yeh CK, Oldewurtel HA, Haider B: Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res. 1981, 49: 1268-1277.
Shehadeh A, Regan TJ: Cardiac consequences of diabetes mellitus. Clin Cardiol. 1995, 18: 301-305.
Brownlee M, Cerami A, Vlassara H: Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988, 318: 1315-1321.
Avendano GF, Agarwal RK, Bashey RI, Lyons MM, Soni BJ, Jyothirmayi GN, Regan TJ: Effects of glucose intolerance on myocardial function and collagen-linked glycation. Diabetes. 1999, 48: 1443-1447.
Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P: An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000, 97: 2809-2813.
Takahashi N, Iwasaka T, Sugiura T, Hasegawa T, Tarumi N, Kimura Y, Kurihara S, Onoyama H, Inada M: Left ventricular regional function after acute anterior myocardial infarction in diabetic patients. Diabetes Care. 1989, 12: 630-635.
Iwasaka T, Takahashi N, Nakamura S, Sugiura T, Tarumi N, Kimura Y, Okubo N, Taniguchi H, Matsui Y, Inada M: Residual left ventricular pump function after acute myocardial infarction in NIDDM patients. Diabetes Care. 1992, 15: 1522-1526.
Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ: Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996, 28: 1661-1669.
van Hoeven KH, Factor SM: A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990, 82: 848-855.
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR: Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991, 83: 778-786.
Griffin BP, Shah PK, Ferguson J, Rubin SA: Incremental prognostic value of exercise hemodynamic variables in chronic congestive heart failure secondary to coronary artery disease or to dilated cardiomyopathy. Am J Cardiol. 1991, 67: 848-853.
Anguita M, Arizon JM, Bueno G, Latre JM, Sancho M, Torres F, Gimenez D, Concha M, Valles F: Clinical and hemodynamic predictors of survival in patients aged <65 years with severe congestive heart failure secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1993, 72: 413-417.
Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R: Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993, 87: VI5-16.
de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM: Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998, 32: 948-954.
Follath F, Cleland JG, Klein W, Murphy R: Etiology and response to drug treatment in heart failure. J Am Coll Cardiol. 1998, 32: 1167-1172.
Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R: Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001, 103: 934-940.
Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L: Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000, 102: 611-616.
Hearse DJ, Stewart DA, Chain EB: Diabetes and the survival and recovery of the anoxic myocardium. J Mol Cell Cardiol. 1975, 7: 397-415.
Ingebretsen CG, Moreau P, Hawelu-Johnson C, Ingebretsen WR: Performance of diabetic rat hearts: effects of anoxia and increased work. Am J Physiol. 1980, 239: H614-20.
Mokuda O, Sakamoto Y, Ikeda T, Mashiba H: Effects of anoxia and low free fatty acid on myocardial energy metabolism in streptozotocin-diabetic rats. Ann Nutr Metab. 1990, 34: 259-265.
Savabi F, Kirsch A: Altered functional activity and anoxic tolerance in diabetic rat isolated atria. Arch Biochem Biophys. 1990, 279: 183-187.
Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991, 325: 293-302.
Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992, 327: 685-691.
Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN: Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002, 40: 1414-1421.
Erdmann E, Lechat P, Verkenne P, Wiemann H: Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail. 2001, 3: 469-479.
Dries DL, Domanski MJ, Waclawiw MA, Gersh BJ: Effect of antithrombotic therapy on risk of sudden coronary death in patients with congestive heart failure. Am J Cardiol. 1997, 79: 909-913.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC: The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996, 335: 1001-1009.
Kjekshus J, Pedersen TR, Olsson AG, Faergeman O, Pyorala K: The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail. 1997, 3: 249-254.
Collaborative overview of randomised trials of antiplatelet therapy – I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. Bmj. 1994, 308: 81-106.
Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G: Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997, 20: 614-620.
Haffner SM: Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998, 21: 160-178.
Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M: Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983, 68: 785-795.
Pigott JD, Kouchoukos NT, Oberman A, Cutter GR: Late results of surgical and medical therapy for patients with coronary artery disease and depressed left ventricular function. J Am Coll Cardiol. 1985, 5: 1036-1045.
Bounous EP, Mark DB, Pollock BG, Hlatky MA, Harrell FE, Lee KL, Rankin JS, Wechsler AS, Pryor DB, Califf RM: Surgical survival benefits for coronary disease patients with left ventricular dysfunction. Circulation. 1988, 78: 1151-1157.
Elefteriades JA, Tolis G, Levi E, Mills LK, Zaret BL: Coronary artery bypass grafting in severe left ventricular dysfunction: excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol. 1993, 22: 1411-1417.
Baker DW, Jones R, Hodges J, Massie BM, Konstam MA, Rose EA: Management of heart failure. III. The role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. Jama. 1994, 272: 1528-34.
Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease: the Bypass Angioplasty Revascularization Investigation (BARI). Circulation. 1997, 96: 1761-9.
O'Neill WW: Multivessel balloon angioplasty should be abandoned in diabetic patients!. J Am Coll Cardiol. 1998, 31: 20-2.
Weintraub WS, Stein B, Kosinski A, Douglas JS, Ghazzal ZM, Jones EL, Morris DC, Guyton RA, Craver JM, King SB: Outcome of coronary bypass surgery versus coronary angioplasty in diabetic patients with multivessel coronary artery disease. J Am Coll Cardiol. 1998, 31: 10-19.
Detre KM, Guo P, Holubkov R, Califf RM, Sopko G, Bach R, Brooks MM, Bourassa MG, Shemin RJ, Rosen AD: Coronary revascularization in diabetic patients: a comparison of the randomized and observational components of the Bypass Angioplasty Revascularization Investigation (BARI). Circulation. 1999, 99: 633-640.
Kuntz RE: Importance of considering atherosclerosis progression when choosing a coronary revascularization strategy: the diabetes-percutaneous transluminal coronary angioplasty dilemma. Circulation. 1999, 99: 847-851.
Seven-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) by treatment and diabetic status. J Am Coll Cardiol. 2000, 35: 1122-1129.
Van Belle E, Bauters C, Hubert E, Bodart JC, Abolmaali K, Meurice T, McFadden EP, Lablanche JM, Bertrand ME: Restenosis rates in diabetic patients: a comparison of coronary stenting and balloon angioplasty in native coronary vessels. Circulation. 1997, 96: 1454-1460.
Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, Neumann FJ, Schomig A: Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998, 32: 1866-1873.
Bhatt DL, Marso SP, Lincoff AM, Wolski KE, Ellis SG, Topol EJ: Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol. 2000, 35: 922-928.
Topol EJ, Mark DB, Lincoff AM, Cohen E, Burton J, Kleiman N, Talley D, Sapp S, Booth J, Cabot CF: Outcomes at 1 year and economic implications of platelet glycoprotein IIb/IIIa blockade in patients undergoing coronary stenting: results from a multicentre randomised trial. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. Lancet. 1999, 354: 2019-2024.
Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G: A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002, 346: 1773-1780.
Bibra H, Hansen A, Dounis V, Bystedt T, malmberg K, Ryden L: Diastolic myocardial function and myocardial microvasculature reserve improve with intense insulin treatment in type 2 diabetic patients. Circulation. 2001, 104 (Suppl II): 369.
The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002, 25: 2165-2171.
MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002, 360: 7-22.
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000, 342: 145-153.
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998, 317: 703-713.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bauters, C., Lamblin, N., Mc Fadden, E.P. et al. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol 2, 1 (2003). https://doi.org/10.1186/1475-2840-2-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-2-1