Abstract
Background
Limited data are available regarding the cost-effectiveness of preventative therapies for postmenopausal women with osteopenia. The objective of the present study was to evaluate the cost-effectiveness of raloxifene, alendronate and conservative care in this population.
Methods
We developed a microsimulation model to assess the incremental cost and effectiveness of raloxifene and alendronate relative to conservative care. We assumed a societal perspective and a lifetime time horizon. We examined clinical scenarios involving postmenopausal women from 55 to 75 years of age with bone mineral density T-scores ranging from -1.0 to -2.4. Modeled health events included vertebral and nonvertebral fractures, invasive breast cancer, and venous thromboembolism (VTE). Raloxifene and alendronate were assumed to reduce the incidence of vertebral but not nonvertebral fractures; raloxifene was assumed to decrease the incidence of breast cancer and increase the incidence of VTEs. Cost-effectiveness is reported in $/QALYs gained.
Results
For women 55 to 60 years of age with a T-score of -1.8, raloxifene cost approximately $50,000/QALY gained relative to conservative care. Raloxifene was less cost-effective for women 65 and older. At all ages, alendronate was both more expensive and less effective than raloxifene. In most clinical scenarios, raloxifene conferred a greater benefit (in QALYs) from prevention of invasive breast cancer than from fracture prevention. Results were most sensitive to the population's underlying risk of fracture and breast cancer, assumed efficacy and costs of treatment, and the discount rate.
Conclusion
For 55 and 60 year old women with osteopenia, treatment with raloxifene compares favorably to interventions accepted as cost-effective.
Similar content being viewed by others
Background
Osteoporosis has been operationally defined as a bone mineral density (BMD) at least 2.5 standard deviations below that seen in young healthy women (T-score < -2.5) and osteopenia as a BMD T-score between -1 and -2.5[1]. Although the risk of fracture is greater in postmenopausal women with osteoporosis than with osteopenia, there are far more postmenopausal women with BMD values in the normal or osteopenic range[2]. As a result, more fractures occur in women with osteopenia than with osteoporosis [3–6]. Guidelines generally agree that women with osteoporosis should receive pharmacotherapy [7–9] for fracture prevention but consensus has not been reached for the management of women with osteopenia. With up to 50% of postmenopausal women in the US estimated to have osteopenia,[2] the optimal approach to fracture prevention in these women is a question of vital public health interest.
Conservative care for osteopenic women includes weight-bearing exercise and calcium/vitamin D supplementation. Pharmacological treatment options include bisphosphonates and raloxifene. The effects of bisphosphonates are largely limited to bone tissue due to their long-term incorporation into the bone matrix[10]. The selective estrogen receptor modulator (SERM) raloxifene is indicated for the prevention and treatment of osteoporosis and is also associated with multiple extra-skeletal effects[11]. Raloxifene differs from bisphosphonates and from other SERMs in that the results of large clinical trials have demonstrated that it reduces the incidence of both vertebral fractures and breast cancer [12–14]. An important adverse event associated with raloxifene is an increased incidence of venous thromboembolism[15, 16]. Selecting a bisphosphonate, raloxifene, or conservative management for women with osteopenia requires consideration of the benefits, risks, and costs of each option.
A recent study concluded that alendronate, a commonly prescribed bisphosphonate, was not cost-effective relative to conservative care for osteopenic women in the absence of additional risk factors for fracture[17]. The effectiveness and cost-effectiveness of raloxifene in this population have yet to be established. To that end, we developed a decision analytic model to examine the effectiveness and cost-effectiveness of raloxifene and alendronate relative to conservative care for postmenopausal women with low bone mass who have not yet experienced a fracture.
Methods
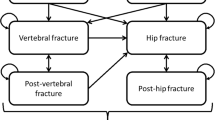
We developed a microsimulation model to assess cost-effectiveness from a societal perspective. The structure of the model and additional methodological details are included in an additional file [see Additional File 1]. We examined clinical scenarios including postmenopausal women without any pre-existing fractures, ranging in starting age from 55 to 75 with BMD T-scores ranging from -1.0 to -2.4 at the femoral neck. For each age and T-score combination, a patient's risk of breast cancer was assumed in the base case to be equal to the population mean and varied in sensitivity analyses. Three management strategies, 5 years of raloxifene, 5 years of alendronate, or no drug therapy, were considered. Patients were assumed to be 100% compliant with therapy but this assumption was varied in sensitivity analyses. All patients were assumed to be receiving supplemental calcium and vitamin D. In each cycle a patient may die or incur any one or more of four fragility fracture types (hip, vertebral, wrist, and other), breast cancer, and/or a venous thromboembolism (VTE). Cohorts of 100,000 women were simulated until age 100 or death. Age-dependent mortality was modeled using 2002 data[18]. Health states were assigned utilities, reflecting patient preferences, from the literature and effectiveness was assessed in quality-adjusted life years (QALYs). Cost-effectiveness was assessed in terms of dollars per event avoided and dollars per QALY. Costs and outcomes were discounted at a 3% annual rate in the base case. Key parameters and assumptions used in the model are summarized in Table 1 and additional information is available in the technical appendix.
Event incidence rates
Age and T-score dependent incidences for spine (both radiographic and clinical), hip, wrist and other fractures were calculated from published data[19]. We assumed that 35% of spine fractures would be clinically apparent[20]. Post-fracture risk multipliers were used to calculate increased rates for subsequent fractures[21]. Following a hip fracture, 23% of the observed increase in mortality at age 60 was assumed to be attributable to the hip fracture event[22]. Fracture reduction efficacy for subgroups of women with T-scores in the osteopenic range have been reported for both raloxifene[23] and alendronate[24]. While there is disagreement about whether alendronate reduces risk of vertebral fracture in osteopenic women [24–27], we assumed that the vertebral fracture risk reduction with alendronate that was reported for women with osteoporosis[25] also applied to women with osteopenia. Neither therapy has demonstrated efficacy in preventing nonvertebral fractures in osteopenic women [23–25]. After treatment cessation, residual fracture reduction benefits were phased out linearly over five years.
Five-year risks of invasive breast cancer were obtained from the Surveillance, Epidemiology, and End Results database[28]. Stage at diagnosis was calculated by pooling 1996–2000 with 2001–2002 data available from the Centers for Disease Control[29]. Age-dependent breast cancer mortalities were computed by stage at diagnosis. The effect of raloxifene on reducing the incidence of invasive breast cancer was assumed to begin in the second year[30] and phase out over five years after raloxifene treatment ends. Alendronate was assumed not to affect the risk of breast cancer.
The incidence of VTE in 67 year old women eligible for and receiving raloxifene treatment was obtained from the placebo group of the MORE trial[15] and extrapolated to other ages[31]. Recurrence rates for VTE were 8%[32] in the first year and 2% in all subsequent years[33]. We assumed 5% of VTEs were fatal[32]. Raloxifene was assumed to confer a 6.2-fold increase in VTE incidence during the first 2 years of raloxifene treatment, with a return to baseline beginning in the 3rd year of therapy[15, 16]. If a patient who received raloxifene experienced a VTE or developed breast cancer, treatment was immediately stopped.
Costs
Medication costs for alendronate and raloxifene reflect April 2006 Net Wholesale Price (NWP)[34]. Other cost estimates were obtained from published literature [35–39] and inflated to April 2006 US dollars using the healthcare component of the Consumer Price Index.(Table 1).
Sensitivity Analyses
Alternative scenarios were considered to understand the importance of the duration of therapy and the patient population's risk of fracture, breast cancer, and VTE. One-way sensitivity analyses were used to determine the influence of other model parameters. In probabilistic sensitivity analyses (presented in the technical appendix), input values were sampled from lognormal distributions for event costs and generalized beta distributions for health state utilities and the relative risk of events with treatment. Using these distributions, 1000 samples of 1000 simulated patients were analyzed.
Results
Base case
For 60 year old women with a T-score in the middle of the osteopenic range (-1.8) and with the population mean risk of breast cancer and VTE, 5 years of treatment with raloxifene has an incremental lifetime discounted cost of $3,726 per patient. The expected net total decrease in clinical vertebral fractures (22.4 fewer cases per 1,000 women) and breast cancers (23.1 fewer cases per 1,000 women) and increase in VTEs (12.1 more cases per 1,000 women) results in a lifetime discounted cost of $111,257 per event avoided. The incremental cost-effectiveness ratio for 60 year old women in the middle of the osteopenic range (T-score = -1.8) is approximately $50,000 per quality-adjusted life year (QALY) relative to conservative care.(Table 2) Similar results were obtained for 55 year old women. Raloxifene was less cost-effective for women starting therapy at age 65 and the cost-effectiveness ratios increased further for 70 and 75 year old women.
Under base case assumptions, alendronate therapy was more expensive and less effective than raloxifene for all combinations of age (55 to 75) and T-score (-1.0 to -2.4) considered. In situations where raloxifene therapy might not be considered a viable treatment option, such as in patients with a history of VTE or with active neoplasms, alendronate costs approximately $113,000/QALY compared to conservative care for 60 year old women with a T-score of -1.8.
The influence of the extra-skeletal effects with raloxifene is summarized in Figure 1. From age 55 to 70, the increase in quality-adjusted life expectancy from the prevention of invasive breast cancers is greater than for fracture prevention. At age 75, the increased risk of VTEs with raloxifene offsets nearly 30% of the effects from fracture and breast cancer prevention.
Relative contributions of the skeletal and extraskeletal effects of raloxifene. The total expected effectiveness is the sum of the contributions from the reduced incidence of vertebral fractures and breast cancers minus the QALYs lost from the increased incidence of venous thromboembolism. Abbreviations: QALY, quality-adjusted life year.
Importance of Patient Population Characteristics
The effect of T-score and risk of breast cancer on raloxifene's cost-effectivness ratio is summarized in Figure 2. At each age, raloxifene was more cost-effective at lower T-scores and higher risk of breast cancer. For women near the threshold of osteoporosis (T-score = -2.4), the incremental cost-effectiveness ratio with raloxifene improved to $36,972, while for women with a T-score of -1.0, it increased to $63,027. For 60 year old women, the cost-effectiveness ratio for raloxifene was less than $50,000/QALY for all osteopenic women with a 5-year risk of breast cancer of greater than 2.25%. For 60 year old women with a 5-year risk of breast cancer of 1.87%, which is the age-dependent population mean risk, the cost-effectiveness ratio for raloxifene was less than $50,000/QALY when the T-score was -1.8 or worse. Raloxifene was cost-saving in 60 year old women with over 4 times the population relative risk of breast cancer (e.g., a woman who has received 2 or more breast biopsies, at least 1 of which resulted in a diagnosis of atypical hyperplasia, and 1 first degree relative with a history of breast cancer) at a T-score of -1.8.
Cost-effectiveness thresholds for raloxifene treatment of postmenopausal women at varying ages, T-score, and risk of breast cancer. Patient populations with a T-score worse or a breast cancer risk greater than that shown at each line would be considered cost-effective at the indicated societal willingess-to-pay. The influence of age is demonstrated by showing the $50,000/QALY threshold at 60 and 70 years of age.
Sensitivity Analyses
If the duration of raloxifene therapy was shortened to 2 years or extended to 7 years, the cost effectiveness ratio increased to $71,442 or $50,944 respectively. Including mortality attributable to vertebral fractures improved the cost-effectiveness ratio to $45,721. Reducing compliance with therapy to 80% or 50% increased the cost-effectiveness ratio to $50,660 or $58,260. Although never shown to do so in a non-osteoporotic population, if alendronate was assumed to reduce the incidence of nonvertebral fractures and the risk of breast cancer was equal to the population mean, then alendronate was found to be more cost-effective than raloxifene in women over the age of 60 with a T-score of -1.9 or lower. If the price of alendronate was reduced by over 50% to $1/day, then the cost-effectiveness ratio compared to conservative care was $46,714 for 60 year old women with a T-score of -1.8. For a SERM with similar efficacy as raloxifene in preventing vertebral fractures but with no effect on breast cancer or VTEs, the cost per QALY was $123,262.
Additional sensitivity analyses are summarized in Figure 3. The results were sensitive to the discount rate and therapy parameters including the efficacy magnitude, efficacy duration after treatment cessation, and medication costs. Patient preferences for post-vertebral fracture and breast cancer states were of moderate importance. The results were less sensitive to the attributable mortality following hip fracture, fracture costs, or any of the VTE parameters.
Univariate sensitivity analyses for raloxifene. The black bars indicate increases and white bars indicate decreases in the incremental cost-effectiveness ratio. The values shown in parentheses correspond to the range of input values. Abbreviations: RRR, relative risk reduction; BrCa, breast cancer; Fx, fracture; VTE, venous thromboembolism.
Discussion
Antiresorptive agents are considered effective in reducing the incidence of fracture and have favorable cost-effectiveness profiles for most postmenopausal women with osteoporosis[40]. Whether these mediations should be used in patients with osteopenia is much less clear even though therapy preserves bone mass and bone structure in these women [7–9]. Because up to 50% of postmenopausal women have osteopenia[2], and more than half of fragility fractures occur in these women[3], this issue is particularly relevant from a health policy perspective. A recent study found that alendronate would likely not be considered cost-effective for the treatment of women with osteopenia diagnosed on the basis of BMD alone[17]. If only fracture prevention is considered, alternative treatment options such as raloxifene, calcitonin, or other bisphosphonates would be expected to have similarly unfavorable cost-effectiveness profiles in women with osteopenia. However, previous decision analyses have found that the extraskeletal effects of raloxifene are important considerations[21, 41–45]. In the current study, assuming a societal willingness-to-pay of $50,000 per QALY, raloxifene would be considered cost-effective for 55 and 60 year old women with T-scores in the lower half of the osteopenic range. For 60 year old women with a 5-year risk of breast cancer that is 50% greater than the population average, raloxifene treatment would cost less than $50,000/QALY for women throughout the osteopenic range (T-score between -1 and -2.5). Examples of additional breast cancer risk factors that would increase the risk of 60 year old women more than 50% include having at least 1 breast biopsy with atypical hyperplasia or at least 1 first degree relative with a history of breast cancer[44]. The extraskeletal effects of raloxifene contributed substantially to its effectiveness, with the reduction in the incidence of breast cancers contributing more to the gain in QALYs compared to fracture prevention for women under the age of 75.
The results for both alendronate and raloxifene were sensitive to assumptions about cost and efficacy. Generic alendronate is currently expected to become available in 2008 and if the price of alendronate drops to $1/day, the cost-effectiveness ratio would be similar to that of raloxifene for 60 year old women with a T-score of -1.8. Consistent with a prior study[17], our findings suggest that at its current price alendronate would not be considered cost-effective for osteopenic women unless it was assumed to decrease the incidence of nonvertebral fractures and restricted to use in women with bone mineral densities nearly in the osteoporotic range.
Previous studies[21, 41, 43–45, 47] have indicated that the cost-effectiveness of raloxifene, which as of 2006 is indicated only for the prevention and treatment of osteoporosis, was driven largely by its associated extraskeletal effects. Although most previous work has focused on patients with osteoporosis[21, 43–45], they have nevertheless demonstrated that the age, risk of fracture, and risk of breast cancer of the target population are key considerations. For example, a model of raloxifene versus placebo found that for 60 year-old women approximately 80% and 20% of overall QALYs gained from raloxifene were attributable to breast cancer prevention and vertebral fracture prevention, respectively, even for an osteoporotic patient population[44]. Another study that modeled healthy postmenopausal women found that raloxifene would be considered a cost-effective alternative to hormone replacement therapy (HRT)[41]. A decision analysis that considered raloxifene, alendronate, or hormone replacement therapy, gains in life expectancy were dependent on a woman's individual risk profile for osteoporosis, breast cancer, and cardiovascular disease[42]. However, the latter two studies were completed prior to the findings from the Women's Health Initiative (WHI) that demonstrated net harm with HRT in relatively healthy postmenopausal women[48]. A comparative economic analysis in a preventative setting that included the WHI results found that raloxifene was more cost-effective than alendronate, while hormone replacement therapy would be predicted to result in net harm[47]. The current study is the first that has incrementally compared raloxifene and alendronate treatment in osteopenic women.
Limitations
First, as with any simulation, the actual clinical situation was simplified to avoid creating an overly complex model. For example, common side effects such as gastrointenstinal upset for bisphosphonates and hot flashes or leg cramps for raloxifene have not been considered. Second, the usefulness of the results depends on the quality of the model inputs and there was substantial uncertainty in several of the parameters. We have addressed this issue through sensitivity analyses but the results of these analyses span a considerable range for some parameters. Third, although the results were dependent on the patient population's risk of developing invasive breast cancer, available methods to calculate the patient's 5-year risk, such as the Gail model[46], are not in widespread use and do not discriminate which individual patients will develop breast cancer[49]. However, for 60 year old women with T-scores in the lower half of the osteopenic range, even when they do not have an elevated risk of breast cancer for their age, raloxifene compared favorably with the commonly used benchmark of $50,000/QALY for a therapy to be considered cost-effective. Finally, the results apply only to postmenopausal women with osteopenia who are otherwise relatively healthy. A recently completed large clinical trial demonstrated that, in women with coronary heart disease (CHD) or an increased risk of CHD, raloxifene was not associated with an increase or decrease in the incidence of coronary events or stroke but there was an increase in stroke mortality[50]. Osteonecrosis of the jaw (ONJ) is a rare but serious adverse event associated with bisphosphonate use[51]. Most, although not all, cases of ONJ have been reported in patients with multiple myeloma or metastatic cancer[51]. We have not included stroke or ONJ in the current model because women at an increased risk for CHD or with cancer would be considered outside the scope of the current study.
These results are not necessarily applicable to other SERMs with different efficacy and safety profiles. For example, tamoxifen has proven efficacy for reducing the incidence of primary and recurrent breast cancer but is also associated with an increased risk of endometrial cancer[52]. In the primary prevention setting, tamoxifen is most cost-effective for younger women at increased risk of breast cancer who have had a hysterectomy[52]. As new SERMs are developed, the specific balance of skeletal (i.e., vertebral and nonvertebral risk reduction) and extraskeletal effects will require new analyses in order to understand the most favorable patient population for each molecule.
An algorithm from the World Health Organization to derive the absolute risk of fracture[54] based on a number of clinical inputs is expected to be released in 2007. The results of the current study, based on T-scores, should be re-calibrated to consider a patient's absolute risk of fracture to be comparable with future cost-effectiveness studies. However, advanced age is one of the most important risk factors for fracture and was one of the variables examined here.
Conclusion
While the present study suggests that the use of raloxifene to prevent fractures in postmenopausal women with osteopenia may be cost-effective on a population level, the optimal treatment decision for an individual patient will depend upon her unique profile of risks for various positive and negative outcomes and values attributed to potential health states, including that of taking a treatment with evident side effects and largely unobservable benefits. As our ability to estimate patient-specific disease risks improves, the importance of integrating these risk assessments and individualizing complex preventative treatment decisions will grow.
References
World Health Organization (WHO Study Group): Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994, 843: 1-129.
Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP: Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997, 12: 1761-1768. 10.1359/jbmr.1997.12.11.1761.
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML: Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004, 164: 1108-1112. 10.1001/archinte.164.10.1108.
Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA: The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006, 17: 1404-1409. 10.1007/s00198-006-0135-9.
Schuit SC, van der KM, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA: Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004, 34: 195-202. 10.1016/j.bone.2003.10.001.
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR, Osteoporotic Fractures Research Group: BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003, 18: 1947-1954. 10.1359/jbmr.2003.18.11.1947.
Hodgson SF, Watts NB, Bilezikian JP, Clarke BL, Gray TK, Harris DW, Johnston CC, Kleerekoper M, Lindsay R, Luckey MM, McClung MR, Nankin HR, Petak SM, Recker RR, Anderson RJ, Bergman DA, Bloomgarden ZT, Dickey RA, Palumbo PJ, Peters AL, Rettinger HI, Rodbard HW, Rubenstein HA, AACE Osteoporosis TF: American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003.[erratum appears in Endocr Pract. 2004 Jan-Feb;10(1):90]. Endocr Pract. 2003, 9: 544-564.
National Osteoporosis Foundation: Physician's guide to prevention and treatment of osteoporosis. 2003, Washington, DC: NOF
North American Menopause Society: Management of postmenopausal osteoporosis: position statement of the North American Menopause Society. Menopause. 2002, 9: 84-101.
Russell RG: Bisphosphonates: from bench to bedside. Ann NY Acad Sci. 2006, 1068: 367-401. 10.1196/annals.1346.041.
Draper MW: The role of selective estrogen receptor modulators (SERMs) in postmenopausal health. Ann NY Acad Sci. 2003, 997: 373-377. 10.1196/annals.1290.040.
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC: The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation.[erratum appears in JAMA 1999 Dec 8;282(22):2124]. JAMA. 1999, 281: 2189-2197. 10.1001/jama.281.23.2189.
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud K, Avioli LV, Lips P, Cummings SR: Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA. 1999, 282: 637-645. 10.1001/jama.282.7.637.
Vogel V, Costantino J, Wickerham D, Cronin W, Cecchini R, Atkins J, Bevers T, Fehrenbacher L, Pajon E, Wade J, Robidoux A, Margolese R, James J, Lippman SM, Runowicz C, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N, for the National Surgical Adjuvant Breast and Bowel Project (NSABP): Effects of Tamoxifen vs Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease Outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006, 295: 2727-2741. 10.1001/jama.295.23.joc60074.
Grady D, Ettinger B, Moscarelli E, Plouffe L, Sarkar S, Ciaccia A, Cummings S, for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators: Safety and Adverse Effects Associated with Raloxifene: Multiple Outcomes of Raloxifene Evaluation. Obstet Gynecol. 2004, 104: 837-844.
Martino S, Disch D, Dowsett SA, keech CA, Mershon J: Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin. 2005, 21: 1441-1452. 10.1185/030079905X61839.
Schousboe JT, Nyman JA, Kane RL, Ensrud KE: Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med. 2005, 142: 734-741.
Vital Statistics of the United States: Mortality. [http://www.cdc.gov/nchs/data/dvs/mortfinal2002_workipt1.pdf]
National Osteoporosis Foundation: Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Osteoporos Int. 1998, 8: S1-S80. 10.1007/s001980050040.
Melton LJ, Lane AW, Cooper C, Eastell R, O'Fallon WM, Riggs BL: Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993, 3: 113-119. 10.1007/BF01623271.
Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J: A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005, 9.
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK: The components of excess mortality after hip fracture. Bone. 2003, 32: 468-473. 10.1016/S8756-3282(03)00061-9.
Kanis JA, Johnell O, Black DM, Downs RW, Sarkar S, Fuerst T, Secrest RJ, Pavo I: Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone. 2003, 33: 293-300. 10.1016/S8756-3282(03)00200-X.
Quandt SA, Thompson DE, Schneider DL, Nevitt MC, Black DM, Fracture Intervention Trial Research Group: Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of-1.6 to -2.5 at the femoral neck: the Fracture Intervention Trial. Mayo Clin Proc. 2005, 80: 349.
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ: Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998, 280: 2077-2082. 10.1001/jama.280.24.2077.
Mikhail N, Cope D: Alendronate and vertebral fracture risk.[comment, see author reply]. Mayo Clin Proc. 2005, 80: 1236.
Seeman E: Alendronate and vertebral fracture risk.[comment, see author reply]. Mayo Clin Proc. 2005, 80: 1236.
SEER 13 Registries for 2000–2002. Probability of Developing Cancer Breast Cancer by Race. [http://canques.seer.cancer.gov]
National Center for Chronic Disease Prevention and Health Promotion. National Breast and Cervical Cancer Early Detection Program. Breast Cancer Stage at Time of Diagnosis. [http://www.cdc.gov/cancer/nbccedp/bccpdfs/national_report.pdf]
Cauley J, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, Farrerons J, Karasik A, Mellstrom D, Ng KW, Stepan JJ, Powles TJ, Morrow M, Costa A, Silfen SL, Walls EL, Schmitt H, Muchmore DB, Jordan VC: Continued Breast Cancer Risk Reduction in Postmenopausal Women Treated with Raloxifene: 4-year Results from the MORE Trial. Breast Cancer Res Treat. 2001, 65: 125-134. 10.1023/A:1006478317173.
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ: Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998, 158: 585-593. 10.1001/archinte.158.6.585.
Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR: Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004, 117: 19-25. 10.1016/j.amjmed.2004.01.018.
Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ: Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000, 160: 761-768. 10.1001/archinte.160.6.761.
Drug average wholesale price. [http://www.analysource.com]
de Lissovoy G, Yusen RD, Spiro TE, Krupski WC, Champion AH, Sorensen SV: Cost for inpatient care of venous thrombosis: a trial of enoxaparin vs standard heparin. Arch Intern Med. 2000, 160: 3160-3165. 10.1001/archinte.160.20.3160.
Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ: Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002, 13: 323-330. 10.1007/s001980200033.
Knight KK, Wong J, Hauch O, Wygant G, Aguilar D, Ofman JJ: Economic and utilization outcomes associated with choice of treatment for venous thromboembolism in hospitalized patients. Value Health. 2005, 8: 191-200. 10.1111/j.1524-4733.2005.04026.x.
Legorreta AP, Brooks RJ, Leibowitz AN, Solin LJ: Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med. 1996, 156: 2197-2201. 10.1001/archinte.156.19.2197.
Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ: Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002, 50: 1644-1650. 10.1046/j.1532-5415.2002.50455.x.
Fleurence RL, Iglesias CP, Torgerson DJ: Economic evaluations of interventions for the prevention and treatment of osteoporosis: a structured review of the literature. Osteoporos Int. 2006, 17: 29-40. 10.1007/s00198-005-1943-z.
Armstrong K, Chen TM, Albert D, Randall TC, Schwartz JS: Cost-effectiveness of raloxifene and hormone replacement therapy in postmenopausal women: impact of breast cancer risk. Obstet Gynecol. 2001, 98: 996-1003. 10.1016/S0029-7844(01)01624-6.
Col NF, Pauker SG, Goldberg RJ, Eckman MH, Orr RK, Ross EM, Wong JB: Individualizing therapy to prevent long-term consequences of estrogen deficiency in postmenopausal women. Arch Intern Med. 1999, 159: 1458-1466. 10.1001/archinte.159.13.1458.
Goeree R, Blackhouse G, Adachi J: Cost-effectiveness of alternative treatments for women with osteoporosis in Canada. Curr Med Res Opin. 2006, 22: 1425-1436. 10.1185/030079906X115568.
Kanis JA, Borgstrom F, Johnell O, Oden A, Sykes D, Jonsson B: Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporos Int. 2005, 16: 15-25. 10.1007/s00198-004-1688-0.
Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK: Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate. Med Decis Making. 2006, 26: 194-206. 10.1177/0272989X06286478.
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ: Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989, 81: 1879-1886. 10.1093/jnci/81.24.1879.
Mullins CD, Ohsfeldt RL: Modeling the annual costs of postmenopausal prevention therapy: raloxifene, alendronate, or estrogen-progestin therapy. J Manag Care Pharm. 2003, 9: 150-158.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002, 288: 321-333. 10.1001/jama.288.3.321.
Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA: Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001, 93: 358-366. 10.1093/jnci/93.5.358.
Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Raloxifene Use for The Heart (RUTH) Trial Investigators: Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006, 355: 125-137. 10.1056/NEJMoa062462.
Woo SB, Hellstein JW, Kalmar JR: Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006, 144: 753-761.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N: Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998, 90: 1371-1388. 10.1093/jnci/90.18.1371.
Cykert S, Phifer N, Hansen C: Tamoxifen for breast cancer prevention: a framework for clinical decisions. Obstet Gynecol. 2004, 104: 433-442.
Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, Melton L, Oden A, Papapoulos S, Pols H, Rizzoli R, Silman A, Tenenhouse A, International Osteoporosis Foundation, National Osteoporosis Foundation: A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002, 13: 527-536. 10.1007/s001980200069.
Macran S, Weatherly H, Kind P: Measuring population health: a comparison of three generic health status measures. Med Care. 2003, 41: 218-231. 10.1097/00005650-200302000-00004.
Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, Jonsson B: The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004, 15: 20-26. 10.1007/s00198-003-1463-7.
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J: Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000, 15: 1384-1392. 10.1359/jbmr.2000.15.7.1384.
Brown RE, Hutton J, Burrell A: Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics. 2001, 19: 1091-1102. 10.2165/00019053-200119110-00003.
Brown RE, Hutton J: Cost-utility model comparing docetaxel and paclitaxel in advanced breast cancer patients. Anticancer Drugs. 1998, 9: 899-907. 10.1097/00001813-199811000-00009.
Launois R, Reboul-Marty J, Henry B, Bonneterre J: A cost-utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996, 10: 504-521.
Norum J, Olsen JA, Wist EA: Lumpectomy or mastectomy? Is breast conserving surgery too expensive?. Breast Cancer Res Treat. 1997, 45: 7-14. 10.1023/A:1005804101106.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6874/7/6/prepub
Acknowledgements
We thank Dr. John T. Schousboe (Park Nicollet Hospitals) for critically reviewing the methodology used in the model and Dr. John L. Stock (Eli Lilly and Company) for providing valuable expertise in osteoporosis and for helpful suggestions in framing the clinical problem. We also thank Timothy M. Klein (Medical Decision Modeling) for providing technical assistance in completing the simulations. Funding for this study was provided by Eli Lilly & Company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Funding for the study was provided by Eli Lilly and Company. ESM, MDR, and JAJ are employees of Eli Lilly and hold stock and stock options in the company. RLO is a former employee of Eli Lilly but does not hold stock or stock options in Eli Lilly.
Authors' contributions
MDR, RK, and LS initiated the study and developed the conceptual framework for the microsimulation model. ESM, LS, RK, RLO and JAJ finalized the design of the model and parameters to be used in the model. LS programmed the microsimulation portion of the model. RK was the lead analyst who obtained and compiled the results. ESM drafted the manuscript with substantial input from JAJ and RWK. Important intellectual contributions on the content of the manuscript were received from MDR, LS, and RLO. All authors approved the final version of the manuscript.
Electronic supplementary material
12905_2006_102_MOESM1_ESM.doc
Additional File 1: Osteopenia_Mansuscript Appendix. The appendix contains additional technical details of the methods and results of the microsimulation model. (DOC 162 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Meadows, E.S., Klein, R., Rousculp, M.D. et al. Cost-effectiveness of preventative therapies for postmenopausal women with osteopenia. BMC Women's Health 7, 6 (2007). https://doi.org/10.1186/1472-6874-7-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6874-7-6