Abstract
Background
Breast cancer today has many established risk factors, both genetic and environmental, but these risk factors by themselves explain only part of the total cancer incidence. We have investigated potential interactions between certain known genetic and phenotypic risk factors, specifically nine single nucleotide polymorphisms (SNPs) and height, body mass index (BMI) and hormone replacement therapy (HRT).
Methods
We analyzed samples from three different study populations: two prospectively followed Swedish cohorts and one Icelandic case–control study. Totally 2884 invasive breast cancer cases and 4508 controls were analysed in the study. Genotypes were determined using Mass spectrometry-Maldi-TOF and phenotypic variables were derived from measurements and/or questionnaires. Odds Ratios and 95% confidence intervals were calculated using unconditional logistic regression with the inclusion of an interaction term in the logistic regression model.
Results
One SNP (rs851987 in ESR1) tended to interact with height, with an increasingly protective effect of the major allele in taller women (p = 0.007) and rs13281615 (on 8q24) tended to confer risk only in non users of HRT (p-for interaction = 0.03). There were no significant interactions after correction for multiple testing.
Conclusions
We conclude that much larger sample sets would be necessary to demonstrate interactions between low-risk genetic polymorphisms and the phenotypic variables height, BMI and HRT on the risk for breast cancer. However the present hypothesis-generating study has identified tendencies that would be of interest to evaluate for gene-environment interactions in independent materials.
Similar content being viewed by others
Background
Genome wide association studies (GWAS), have discovered several new genetic polymorphisms affecting breast cancer risk [1–3]. Even though these individual risk-factors each confer quite small increases in risk, a positive association is seen between the number of risk alleles carried and risk for breast cancer [4, 5].
The phenotypic variables height, body mass index (BMI) and use of hormone replacement therapy (HRT) reflect to varying degrees genetic background and environmental exposure. Both height and BMI have previously been shown to associate with breast cancer [6, 7]. Increase in height has been shown to yield a proportional increase in breast cancer risk and obese women have a greater risk to contract postmenopausal breast cancer. Increased risk is also established for users of HRT [7], which has been speculated to interact with low-risk polymorphisms in the FGFR2 gene [8, 9].
Although there have been investigations on gene-environment interactions in breast cancer [10], this area remains to a large extent unexplored.
The aim of this study was to investigate if height, BMI and HRT modify the genetic predisposition to breast cancer conferred by reported low-risk polymorphisms. For this purpose we had access to two well defined Swedish population based cohorts as well as an Icelandic hospital based case control study, altogether 7738 samples (3016 cases and 4722 controls).
Methods
Study populations
The samples originate from two Swedish independent population based cohorts; the Malmö Diet and Cancer Study (MDCS) from southern Sweden and the North Sweden Health and Disease Study (NSHDS), together comprising 2410 incident cases and 3829 controls. The third sample collection was an Icelandic population-based case control study including 866 cases and 948 controls. Written informed consent was retrieved from all women prior to donating their samples. All cohorts have been described previously [11] and are briefly presented below.
MDCS
The Malmö Diet and Cancer Study (MDCS) is a prospective cohort study initiated in 1991. Totally it comprises 17035 female residents of Malmö Sweden recruited between 1991 and 1996 [12, 13]. By linkage to the national cancer registry until 31st of December 2007, 730 incident cases of invasive breast cancer were identified among MDCS participants. They were matched to 1460 controls from the same cohort according to sex, age (+/− 6 months), and date of sampling at baseline (+/− 2 months). Median age at breast cancer diagnosis was 65 years (range 45–84). Thirty-three cases and 65 controls were ≤50 years of age at time of diagnosis.
The MDCS and the present analyses were approved by the Ethical Committee at Lund University (LU 51–90, Dnr 652/2005 and Dnr 2009/682).
NSHDS
The Northern Sweden Health and Disease Study (NSHDS) include the Västerbotten Intervention Program (VIP), and the Mammography Screening Program (MSP), initiated in 1985 and 1995 respectively. Participants in the VIP are screened at 40, 50 and 60 years of age and mammography screening and blood sampling is performed among women between 50 and 69 years of age [14]. Through linkage with the cancer register up to December 31st, 2008, 1680 prospective cases of invasive breast cancer (median age 56 years, range 27–95) were identified. They were matched to 2314 controls by sex, age (+/− 6 months), and date of sampling at baseline (+/− 2 months), (474 cases and 606 controls ≤50 years of age). Information on HRT use was available for 1420 of these cases.
The NSHDS and the present analyses were approved by the Ethical Committee at Umeå University (Dnr: 2010-147-132 and 07–141).
ICELAND
The Icelandic samples were collected between 1998 and 2006 and represent 45–77% of all Icelandic women with invasive breast cancer diagnosed between 1957 and 2007. The rate of participation varied somewhat depending on the year of diagnosis and was highest between 1999 and 2003 (77%). Unmatched controls were collected between 2000 and 2004, either from women who participated in the population-based cervical or breast cancer screening program and found free of breast cancer or from older women in retirement homes who had not been diagnosed with breast cancer, to generally reflect the ages of the cases. By linkage to the Icelandic cancer registry in 2008 we identified cases diagnosed before 31st of December 2007. Totally 866 cases (median age 55 years, range 22–98, 314 ≤ 50 years) and 948 controls (median age 58 years, range 25–102, 256 ≤ 50 years) had DNA available and were eligible to us.
The use of these samples was approved by the data protection law (200605037), and the Icelandic Science Ethics Committee (VSNb2006050001/03-16 and VSNb2005070008/03-16).
Data collection
Participants in both Swedish cohorts completed a questionnaire providing information about current medication at the time of recruitment. Participants in the NSHDS also provided information about height and weight while a trained nurse at the study centre measured height and weight, for participants in MDCS [15].
The Icelandic women answered questions about height, weight and HRT use when they attended the Detection Cancer Clinic (breast cancer mammography or cervical screening) at the Icelandic Cancer Society. The women answered questions at least every tenth year and the most recent answers were used in the study. For the Icelandic cases only data collected prior to breast cancer diagnosis was used. BMI for all participants was calculated as kg/m2.
SNP selection
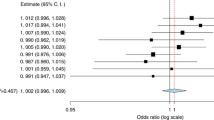
All loci identified by GWAS to be associated with breast cancer and published before June 31st 2007 were initially included in the study [1–3]. Individual SNPs were selected from the publications by Easton et al. and Stacey et al. This primary selection included 10 SNPs, as well as one SNP in CASP8 identified using the candidate gene approach [16]. Two SNPs selected from our own candidate CpG SNP study [11] were also included making a total of 13 SNPs (Figure 1).
Odds Ratios and Confidence Intervals for all SNPs. All 13 primary polymorphisms and their respective OR and p-value in this sample set. Squares represent OR and brackets represent 95% CI for samples adjusted for age and study population. A subset of previously published data [11].
Assay design and genotyping
Eleven SNPs, combined by the SEQUENOM MassARRAY® Designer software in a single multiplex assay were analyzed on a MALDI-TOF mass spectrometer (SEQUENOM MassArray) using standard iPLEX reagents and protocol (SEQUENOM) and 10 ng DNA as PCR template. Primer sets were from Metabion (Martinsried, Germany).
SNPs rs2981582 and rs1045485 were analyzed by a separate TaqMan® “assay by design” genotyping assay on a 7900HT instrument, using Master mix No UNG from Applied Biosystems according to the manufacturer’s instructions. Reaction mixtures (6μL) containing 2 ng of DNA template, primers (rs2981582 forward primer 5′-CAG CAC TCA TCG CCA CTT AAT G-3′, reverse primer 5′-GAC ACC ACT CGG ACT GCT-3′, and probes 5′-VIC-TCT CCG CAA ACA GG-MGB-3′ and 5′-FAM-CTC TCC ACA AAC AGG-MGB-3′) (rs1045485 forward primer 5′-ACC ACG ACC TTT GAA GAG CTT -3′, reverse primer 5′-ACT GTG GTC CAT GAG TTG GTA GAT-3′, and probes 5′-VIC-CCC CAC GAT GAC TG-MGB-3′ and 5′-FAM-CCC CAC CAT GAC TG-MGB-3′) were subjected to two minutes at 50°C and ten minutes at 95°C, followed by 50 PCR cycles of 95°C for 15 seconds and 60°C for one minute.
Three percent of the samples from NSHDS and five percent of the Icelandic samples were included as blinded duplicates for quality control purposes.
Statistical analysis
Individual samples producing results in < 80% of the assays were excluded prior to statistical analyses to eliminate samples with low-quality DNA. Genotype data from control samples were tested for consistency with Hardy-Weinberg equilibrium (HWE) using a χ² p-value cutpoint of 0.001. Unconditional logistic regression was used to measure the independent association between each genotype and breast cancer, with Odds ratios and 95% confidence intervals (CI) estimated for each genotype. Per allele OR (p-trend) was calculated using 0, 1 or 2 copies of the minor allele (a) as a continuous variable. OR and 95% CI were calculated between each phenotypic variable (Height, BMI and HRT) and risk for breast cancer, these results were also age adjusted. Data was then stratified into tertiles according to height (<162 cm, 162–166 am and >166 cm), and into subcategories of BMI according to the WHO guidelines (Normal weight: 18.5-25, Overweight: 25–30 and Obese > 30). For HRT subjects data was stratified according to reported “non use” and “current use”. The current users were further divided into users of only estrogen or combined hormones. OR and 95%CI were calculated for each variable (Height, BMI and HRT) and risk for breast cancer.
A p-value for interaction was estimated for each pair of genotype/phenotype and a value of less than 0.05 was considered statistically significant. As adjustment for multiple comparisons this value was divided by the number of interaction analyses, according to Bonferroni, (8 SNPs x 3 =24) and the new significance threshold was 0.002. All results were adjusted for age and study population.
Results
Of the initial 7738 samples selected for the project 7392 (95.5%) were successfully retrieved and genotyped for ≥ 80% of the SNPs. All SNPs had a genotyping success rate > 94%, with an average of 98.0%. Results of all 200 analyses performed on duplicate samples were in 100% concordance.
Per allele OR for each independent SNP is presented in Figure 1. Ten of the SNPs were significant (p < 0.05) in our material with rs2981852 (FGFR2), rs889312 (MAP3K1) and rs3803662 (TOX3) exhibiting the highest ORs. Two of the SNPs had p-values >0.1 (rs1045485 [CASP8] and rs30099 [5q11]) and were excluded from further analysis.
Three of the SNPs in TOX3 (rs3803662, rs12443621 and rs8051542) exhibited linkage (results not shown) as has previously been reported [1, 4]. Rs12443621 and rs80515442 were therefore excluded from further analysis.
Independent analysis of risk association with each phenotypic variable (height, BMI, HRT) within the entire study population revealed a significantly increased risk of breast cancer for individuals >162 cm compared to shorter women, this association was weakened following age adjustment. No statistical significant correlation between BMI and risk for breast cancer was found in this population. For current use vs. non-use of HRT, a significantly increased risk was seen for users, OR (95% CI) 1.24 (1.08-1.42), which remained after adjustment for age (Table 1).
Stratified analysis and interactions
After stratification by height (as described in materials and methods), one SNP (rs851987) in ESR1 had a p-interaction = 0.007 with height, with an increasingly protective effect of the major allele in taller women, but it did not pass the threshold for multiple comparisons (p = 0.002) (Table 2).
None of the SNPs showed any tendencies towards significant interactions after stratification according to BMI (Table 3).
Following stratification of genotypes according to reported current use or non-use of hormone replacement therapy, rs13281615 (8q24) was significant only in non users of HRT with a p-for interaction of 0.03, indicating borderline significance (Table 4).
Discussion
In this study we have explored interactions between reported genetic risk factors for breast cancer and the three additional established risk factors; height, BMI and HRT in 2884 cases and 4508 controls. The strongest tendency for interaction found was that between height and rs851987 in ESR1, although it did not pass the threshold for multiple comparisons. Taller women carrying the T-allele appeared to have reduced breast cancer risk (p for interaction = 0.007) (Table 2). Rs851987 was described by Harlid et al. [11] and is situated in the far end of the extended promoter region of ESR1, about 3.7 kb 5′ of exon F. Exon F and its promoter were originally described by Thompson et al. [17] and have later been shown to affect the level of ESR1 expression in osteoblastic cells [18, 19]. A potential association between ESR1 and height has been described in another study comprising adult males from two Swedish population cohorts [20]. Mutations in ESR1 have been reported to delay fusion of the epiphyseal plates at puberty [21], and one may speculate that rs851987 either participates in this biological effect or is linked to other causal variants.
One SNP (rs13281615 in 8q4) first described by Easton et al. [1] showed a weak tendency for interaction with use of HRT. The minor allele seems to confer increased breast cancer risk in HRT non-users but no excess risk in current users. The association in non-users is strong with a per-allele OR (95%CI) of 1.20 (1.10-1.31) (p-trend = 6.1 × 10-5) compared to a per-allele OR (95%CI) of 1.08 (1.01-1.15) (p-trend = 0.03) in all users. The SNP is situated in region 8q24 that contains no known genes but is in close proximity to FAM84B (coding for a breast cancer membrane associated protein) and the proto-oncogene MYC. The 8q24 locus has previously been reported to associate with other types of cancer in addition to breast cancer [22] and to be more strongly associated with ER + than ER- tumours [23].
Since the first GWAS on breast cancer was published in 2007 several replication and interaction studies of varying sizes have been published [24–27]. In 2010, a large interaction study comprising 7610 breast cancer cases from the Million Women Study in UK was undertaken and potential interactions between 12 different SNPs and 10 different variables (including height, BMI and HRT) were tested [10]. This study did not find (contrary to previous suggestions) any significant gene-environment interactions. Our study originally included ten of the same polymorphisms as in the Million Women Study (excluding rs1982073 in TGFB1 and rs1800054 in ATM), but also included one additional SNP from Easton et al. [1] and two additional SNPs from our own candidate CpG study [11] (rs7766585 and rs851987 both in ESR1). Although our material is not as large, our study is comprised of three well described study-populations, two of which were prospectively followed for breast cancer incidence using the comprehensive, population-based Swedish Cancer Registry [28]. Thus, our complete case ascertainment and ability to select matched controls from the same study base is likely to have resulted in low risk for selection biases. However, the intervals between data collection, blood sampling, and diagnosis differ substantially between the three different study populations, something that might be considered a limitation of the study.
Considering demographic traits, participants in the MDCS have a slightly higher socioeconomic status than the general population, but as this selection is the same for the study base from which cases and controls are derived, it should not affect the validity of our study [13]. MDCS participants were recruited at age 45–65 years. The exclusion of prevalent cases removes early breast cancer cases from this population. While the NHSDS participants were primarily included from age 40 and upwards, mammography screening had identified some cases as young as 27 years. In Iceland prevalent cases of breast cancer were recruited at varying times after diagnosis, resulting in an exclusion of early lethal cases and older women with other causes of death. As the Icelandic controls were collected later and from the same sample population as the cases there is the possibility of selection bias. Another limitation of our study is the fact that HRT is reported only once (at recruitment) without information about duration. We also lacked information about other risk factors than age, height, BMI, HRT and therefore could not adjust our results for other potential confounders.
Conclusions
Our evaluation of genetic predisposition for breast cancer in relation to three different environmental risk factors found no significant gene-environment interactions. We did find tendencies for certain SNPs to exert an effect on breast cancer risk only in women with certain phenotypes. In particular the potential interaction between height and rs851987 in ESR1 in relation to breast cancer risk could merit further investigation. However, independent studies with many more cases would be needed to verify this finding.
References
Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, et al: Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007, 447: 1087-1093. 10.1038/nature05887.
Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, et al: A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007, 39: 870-874. 10.1038/ng2075.
Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, et al: Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007, 39: 865-869. 10.1038/ng2064.
Reeves GK, Travis RC, Green J, Bull D, Tipper S, Baker K, Beral V, Peto R, Bell J, Zelenika D, Lathrop M: Incidence of breast cancer and its subtypes in relation to individual and multiple low-penetrance genetic susceptibility loci. JAMA. 2010, 304: 426-434. 10.1001/jama.2010.1042.
Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, et al: Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010, 362: 986-993. 10.1056/NEJMoa0907727.
Hunter DJ, Willett WC: Diet, body size, and breast cancer. Epidemiol Rev. 1993, 15: 110-132.
Key TJ, Verkasalo PK, Banks E: Epidemiology of breast cancer. Lancet Oncol. 2001, 2: 133-140. 10.1016/S1470-2045(00)00254-0.
Prentice RL, Huang Y, Hinds DA, Peters U, Pettinger M, Cox DR, Beilharz E, Chlebowski RT, Rossouw JE, Caan B, Ballinger DG: Variation in the FGFR2 gene and the effects of postmenopausal hormone therapy on invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009, 18: 3079-3085. 10.1158/1055-9965.EPI-09-0611.
Rebbeck TR, DeMichele A, Tran TV, Panossian S, Bunin GR, Troxel AB, Strom BL: Hormone-dependent effects of FGFR2 and MAP3K1 in breast cancer susceptibility in a population-based sample of post-menopausal African-American and European-American women. Carcinogenesis. 2009, 30: 269-274.
Travis RC, Reeves GK, Green J, Bull D, Tipper SJ, Baker K, Beral V, Peto R, Bell J, Zelenika D, Lathrop M: Gene-environment interactions in 7610 women with breast cancer: prospective evidence from the Million Women Study. Lancet. 2010, 375: 2143-2151. 10.1016/S0140-6736(10)60636-8.
Harlid S, Ivarsson MI, Butt S, Hussain S, Grzybowska E, Eyfjord JE, Lenner P, Forsti A, Hemminki K, Manjer J, et al: A candidate CpG SNP approach identifies a breast cancer associated ESR1-SNP. Int J Cancer. 2011, 129: 1689-1698. 10.1002/ijc.25786.
Berglund G, Elmstahl S, Janzon L, Larsson SA: The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993, 233: 45-51. 10.1111/j.1365-2796.1993.tb00647.x.
Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, Mattisson I, Berglund G: The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001, 10: 489-499. 10.1097/00008469-200112000-00003.
Pukkala E, Andersen A, Berglund G, Gislefoss R, Gudnason V, Hallmans G, Jellum E, Jousilahti P, Knekt P, Koskela P, et al: Nordic biological specimen banks as basis for studies of cancer causes and control–more than 2 million sample donors, 25 million person years and 100,000 prospective cancers. Acta Oncol. 2007, 46: 286-307. 10.1080/02841860701203545.
Manjer J, Elmstahl S, Janzon L, Berglund G: Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J Public Health. 2002, 30: 103-112.
Cox A, Dunning AM, Garcia-Closas M, Balasubramanian S, Reed MW, Pooley KA, Scollen S, Baynes C, Ponder BA, Chanock S, et al: A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet. 2007, 39: 352-358. 10.1038/ng1981.
Thompson DA, McPherson LA, Carmeci C, deConinck EC, Weigel RJ: Identification of two estrogen receptor transcripts with novel 5′ exons isolated from a MCF7 cDNA library. J Steroid Biochem Mol Biol. 1997, 62: 143-153. 10.1016/S0960-0760(97)00029-0.
Lambertini E, Penolazzi L, Giordano S, Del Senno L, Piva R: Expression of the human oestrogen receptor-alpha gene is regulated by promoter F in MG-63 osteoblastic cells. Biochem J. 2003, 372: 831-839. 10.1042/BJ20021633.
Penolazzi L, Lambertini E, Giordano S, Sollazzo V, Traina G, del Senno L, Piva R: Methylation analysis of the promoter F of estrogen receptor alpha gene: effects on the level of transcription on human osteoblastic cells. J Steroid Biochem Mol Biol. 2004, 91: 1-9. 10.1016/j.jsbmb.2004.02.005.
Dahlgren A, Lundmark P, Axelsson T, Lind L, Syvanen AC: Association of the estrogen receptor 1 (ESR1) gene with body height in adult males from two Swedish population cohorts. PLoS One. 2008, 3: e1807-10.1371/journal.pone.0001807.
Emons J, Chagin AS, Malmlof T, Lekman M, Tivesten A, Ohlsson C, Wit JM, Karperien M, Savendahl L: Expression of vascular endothelial growth factor in the growth plate is stimulated by estradiol and increases during pubertal development. J Endocrinol. 2010, 205: 61-68. 10.1677/JOE-09-0337.
Ghoussaini M, Song H, Koessler T, Al Olama AA, Kote-Jarai Z, Driver KE, Pooley KA, Ramus SJ, Kjaer SK, Hogdall E, et al: Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst. 2008, 100: 962-966. 10.1093/jnci/djn190.
Garcia-Closas M, Hall P, Nevanlinna H, Pooley K, Morrison J, Richesson DA, Bojesen SE, Nordestgaard BG, Axelsson CK, Arias JI, et al: Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008, 4: e1000054-10.1371/journal.pgen.1000054.
Antoniou AC, Sinilnikova OM, McGuffog L, Healey S, Nevanlinna H, Heikkinen T, Simard J, Spurdle AB, Beesley J, Chen X, et al: Common variants in LSP1, 2q35 and 8q24 and breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet. 2009, 18: 4442-4456. 10.1093/hmg/ddp372.
Gorodnova TV, Kuligina E, Yanus GA, Katanugina AS, Abysheva SN, Togo AV, Imyanitov EN: Distribution of FGFR2, TNRC9, MAP3K1, LSP1, and 8q24 alleles in genetically enriched breast cancer patients versus elderly tumor-free women. Cancer Genet Cytogenet. 2010, 199: 69-72. 10.1016/j.cancergencyto.2010.01.020.
Hemminki K, Muller-Myhsok B, Lichtner P, Engel C, Chen B, Burwinkel B, Forsti A, Sutter C, Wappenschmidt B, Hellebrand H, et al: Low-risk variants FGFR2, TNRC9 and LSP1 in German familial breast cancer patients. Int J Cancer. 2010, 126: 2858-2862.
Huijts PE, Vreeswijk MP, Kroeze-Jansema KH, Jacobi CE, Seynaeve C, Krol-Warmerdam EM, Wijers-Koster PM, Blom JC, Pooley KA, Klijn JG, et al: Clinical correlates of low-risk variants in FGFR2, TNRC9, MAP3K1, LSP1 and 8q24 in a Dutch cohort of incident breast cancer cases. Breast Cancer Res. 2007, 9: R78-10.1186/bcr1793.
Barlow L, Westergren K, Holmberg L, Talback M: The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009, 48: 27-33. 10.1080/02841860802247664.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6874/12/17/prepub
Acknowledgements
We thank Anders Dahlin (MDCS), Åsa Ågren (NSHSD) and Holmfridur Hilmarsdottir (UI/ICS) for sample retrieval and handling, Maria Sterner and Liselott Hall at RSKC (Malmö) research facility for technical assistance and the Icelandic Cancer Registry for providing data. This work was supported by the European Union Network of Excellence grant “Cancer Control using Population-based Registries and Biobanks” and the Breast Cancer Network at Lund University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SH had full access to all the data and in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SH and JC are the principal investigators of the study and were responsible for the planning of the study. JD participated in the conception and design of the study and contributed with supervising, funding, and administrative support. MILI participated in designing the study. JEE and PL contributed samples to the study. SH and MILI performed all genetic analysis. SH analyzed the data (data extraction and statistical analysis). SH, JC, JD, SB and JM interpreted the data. SH performed the literature search. SH wrote; JC, JD, JM revised; MILI, SB, JEE, and PL reviewed the paper and contributed suggestions for improvement that were implemented. All authors approved the final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Harlid, S., Butt, S., Ivarsson, M.I. et al. Interactive effect of genetic susceptibility with height, body mass index, and hormone replacement therapy on the risk of breast cancer. BMC Women's Health 12, 17 (2012). https://doi.org/10.1186/1472-6874-12-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6874-12-17