Abstract
Background
Attempts at gene therapy for the pulmonary manifestations of Cystic Fibrosis have relied mainly on airway delivery. However the efficiency of gene transfer and expression in the airway epithelia has not reached therapeutic levels. Access to epithelial cells is not homogenous for a number of reasons and the submucosal glands cannot be reached via the airways.
Presentation
We propose to inject gene delivery vectors directly into bronchial arteries combined with pre-delivery of vascular endothelial growth factor to increase vascular endothelial permeability and post-delivery flow reduction by balloon occlusion. Thus it may be possible to reach mucous secreting cells of the bronchial luminal epithelium and the submucosal glands in an increased and homogenous fashion.
Testing
This combination of techniques to the best of our knowledge has not previously been investigated, and may enable us to overcome some of the current limitations to gene therapy for Cystic Fibrosis.
Similar content being viewed by others
Background
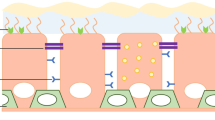
Cystic fibrosis (CF) is the commonest, severe autosomal recessive disease in the UK, occurring in one child out of every 2500 new-borns. The disease is caused by mutations in the Cystic Fibrosis Transmenbrane Conductance Regulator (CFTR) gene, leading to production of defective CFTR protein, which disrupts chloride transport resulting in markedly impaired water fluxes across various epithelial layers. This leads to 'sticky' mucous secretions which obstruct the secretory glands of the lungs, digestive tract and other organs. In spite of considerable advances in the treatment of the intestinal symptoms, comparatively less progress has been achieved in the management of CF lung disease.
Airway Delivery
To date, almost all attempts at gene therapy for the pulmonary manifestations of CF have relied on vector delivery via the airways. This work in animal models and human trials has demonstrated the ability to deliver genes by non-viral and viral gene transfer vectors to the airway epithelia by methods of inhalation, spray or bronchoscopic delivery. Although these investigations demonstrated that the general strategy of gene therapy for CF is hopeful, they also showed that the efficiency of even the presently most effective vector system, adenovirus, is still disappointingly low [2–9]. For adenovirus, this inefficiency is specifically due to the scarcity of the adenovirus fibre knob binding receptors: coxsackievirus and adenovirus receptor (CAR) and the absence of avb3 and avb5 integrins, on the apical side of differentiated airway epithelia [10–16]. In addition, the glyoxalin components (particularly tethered mucins) of the apical cell surface of the airway epithelia appear to be strong barriers to adenoviral gene transfer [11] and these are ultimately secreted in the 'sticky' mucous, which is also an effective hindrance for access to vectors. Furthermore the deeper submucosal glands which express high levels of CFTR [17] play a critical role in the development of respiratory disease in CF [18] and yet are almost impossible to reach from the airway lumen [19]. The basal cells which are important progenitors of the bronchial epithelium are also not exposed to the airway lumen and are concentrated more heavily in the larger bronchi.
Intravenous Delivery
Considering the non-airway studies; intravenous vector delivery has been studied in mice but has resulted predominantly in alveolar gene transfer and only low level gene delivery to the upper airway epithelia [20]. This is because the pulmonary arterial route serves the areas of the lung between the terminal bronchioles and alveoli, rather than the bronchii where mucous is mainly produced. Using thoracotomy and pulmonary arteriotomy of the left pulmonary artery in rats, again disappointingly low levels of transgene expression (1% to 8% of total left lung cells) have been found using an adenoviral vector. Of these cells, between 2% and 50% were airway epithelia and between 38% to 94% were alveolar epithelial cells depending on the animal. Lemarchand et al. have infused adenoviral vectors (Ad.RSVβ gal) directly into the pulmonary circulation of sheep [21]. After 15 minutes of dwell time, where both pulmonary arteries and veins were occluded, they unexpectedly found β-Gal expression in bronchial and alveolar epithelium including the epithelium of submucosal glands. They postulated gene delivery to have occurred via the numerous anastomoses between the pulmonary and bronchial circulations, but were unable to determine how the vectors had crossed the epithelial basal membrane, though to be relatively impermeable. The expression was patchy and did not occur in all animals (none in 4 of 17). A direct bronchial artery approach may however improve on this initial success particularly with the use of adjuvant techniques to increase vascular permeability. These pathophysiological considerations highlight why delivery of vectors targeting the systemic arterial route, via the bronchial arteries to the mucous producing bronchial airways should be attempted to overcome the current limitations of gene therapy vector delivery for CF lung disease. To the best of our knowledge this specific approach has not been investigated however we are aware of a number of other groups who are also planning experiments along these lines, but have yet to address issues of permeability of the vascular barriers.
Presentation of the Hypothesis
We suggest delivery of gene transfer vectors into the bronchial arteries (initially in a sheep model) with pre-delivery of vascular endothelial growth factor and post delivery sub-total flow occlusion, to enhance vascular permeability and increase vector dwell time respectively. We suggest this may allow the vectors to reach the bronchial epithelium and sub-mucosal glands efficiently and with a high degree of homogeneous gene transfer. This should enable therapeutic expression levels of CFTR. In addition we assume that the so far unidentified stem cells of the respiratory epithelium which reside preferentially in the cell layer close to the basal lamina of the bronchial epithelia will also be transfected.
Testing the Hypothesis: Delivery via Bronchial Arteries
A non-animal ex vivo model of a complete lung is currently unavailable. Of the possible animal models, sheep have lungs closest to human anatomy and physiology [22, 23] and have been extensively used for the study of the bronchial circulation physiology, tolerating vascular studies well in experienced hands. In sheep, the bronchial artery arises as a single large carinal vessel that supplies 80% of the systemic flow to both lungs. The ostial diameter of this artery varies from 1 – 6 mm and would accept 5 French guiding catheters for vector delivery. The artery descends into the lung supplying blood via branches to the main and minor bronchi up to the distal terminal bronchioles providing a rich peribronchial capillary plexus of thin vessels (5–20 μm in diameter) which lies just below the respiratory epithelium in the sub-mucosa surrounding the mucous secreting glands. At the microscopic level the bronchial artery branches are histologically distinct from their pulmonary arterial counterparts in that they have no clearly defined external elastic lamina. The endothelium of these arterioles is of the fenestrated type and investigators have demonstrated the passage of fluid from the arterioles into the bronchial mucosa, as well as the passage of neutrophils across the capillaries via active transport through endothelial cell junctions. These anatomical factors highlight why vectors delivered via the bronchial arteries should have an excellent chance of reaching the sub-mucosal layer of all bronchii and thereby all target cells.
Applicability of the Bronchial Artery approach in humans
Bronchial arterial catheterisation in humans via a percutaneous approach has been practiced for 33 years, initially for direct chemotherapy treatment for bronchial malignancies and then for the embolisation of patients with severe haemoptysis. The safety issues that have arisen during these procedures include inadvertent occlusion or embolisation into the anterior spinal artery which in a small percentage (highest 5%) of humans arises from one of the branches of the bronchial arteries [24, 25]. Catheterisation itself carries a less than 1% mortality in human coronary angioplasty series with well defined risks. Finally the issues of oedema due to arterial engorgement and immune response to the vectors must be quantified at various dosages and volumes by histological examination. The issue of systemic spill over of vector material into the cardiac atria and thereafter into the systemic circulation would need to be evaluated by sampling local and distant organs. Nevertheless bronchial artery catheterisation is an established technique amongst vascular interventionists and the extension of this hypothesis for cystic fibrosis patients would be feasible particularly as their bronchial arteries are considerably dilated [26]. Progress is also continually being made in minimally invasive cardiac interventions in the fetus and therefore the delivery to the fetal bronchial arteries may become feasible in the future [27].
Adjuvant techniques for improving Vascular Permeability
Despite the anatomical advantages, the following adjuvant techniques may be needed to increase transvascular vector delivery.
Increase Dwell Time
Maintaining bronchial artery sub-total occlusion for brief periods would increase vector transfer to the bronchial tissue and a reduced total flow mediated wash out of the vector into the heart. Such flow interventions using angioplasty balloons to reduce forward flow can be maintained for lengthy periods by choosing the appropriate balloon diameter without tissue or vessel injury. A degree of interventional experience is needed here.
Increased Permeability of the Vascular Endothelium
Histamine increases permeability of both the bronchial and pulmonary vessels at the level of the microcirculation beds, probably acting on post-capillary venules where intracellular junctions are opened to cause this effect [28–30]. Vascular Endothelial Growth Factor increases microvessel permeability to plasma proteins with 50,000 times higher potency than histamine [31]. This growth factor causes early hyperpermeability probably by vesico-vacuolar organelles and transcellular pores [32] within minutes. These vesico-vacuolar organelles span a range of sizes from 60 to well over 300 nm [33]. Permeability is increased to the extent of allowing erythrocyte and colloidal carbon to pass, which are far greater in size than adenoviral vectors (300 nm). Although this may cause oedema, clinically in work by Isner and colleagues [34] on muscular administration of vascular endothelial growth factor, this was rarely significant and responded to Frusemide.
Vectors for Gene Delivery through the Arterial route
Ideally one would wish to use a vector system which could provide long term or even permanent gene delivery and preferentially also target the stem cell population of the airway epithelia. This would provide a basis for constant renewal of epithelium with a corrected cell population and obviate the need for repeated gene delivery. Retroviral, lentiviral and adeno-associated viral vectors could fulfil this requirement but relatively little is presently known about their ability to infect the airway epithelia in vivo.
Implications of the Hypothesis
On a practical basis, to demonstrate proof of principle for successful and consistent vector delivery to the target cells from the basolateral side by delivery via the bronchial arteries an adenovirus vector expressing a marker gene such as beta-galactosidase could be used as first choice. At a later stage CFTR expressing vector systems would be used. New international developments such as the elimination of all the adenovirus structural genes from this vector system are most likely to overcome to a large extent its present immunological problems. However, long term correction of cystic fibrosis will most likely require application of one of the above mentioned integrating vector systems.
Abbreviations
- CF:
-
Cystic Fibrosis
- CFTR:
-
Cystic Fibrosis Transmenbrane Conductance Regulator
References
Bigger BW, Coutelle C: Perspectives on Gene Therapy for Cystic Fibrosis Airway Disease. BioDrugs. 2001, 15: 615-634.
Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ: Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993, 75: 207-16.
Caplen NJ, Alton EW, Middleton PG, et al: Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis [see comments] [published erratum appears in Nat Med 1995 Mar;1(3):272]. Nat.Med. 1995, 1: 39-46.
Crystal RG, McElvaney NG, Rosenfeld MA, et al: Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis [see comments]. Nat.Genet. 1994, 8: 42-51.
Boucher RC, Knowles MR, Johnson LG, et al: Gene therapy for cystic fibrosis using E1-deleted adenovirus: a phase I trial in the nasal cavity. The University of North Carolina at Chapel Hill. Hum.Gene Ther. 1994, 5: 615-39.
Hay JG, McElvaney NG, Herena J, Crystal RG: Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum.Gene Ther. 1995, 6: 1487-96.
Knowles MR, Hohneker KW, Zhou Z, et al: A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis [see comments]. N.Engl.J.Med. 1995, 333: 823-31. 10.1056/NEJM199509283331302.
Zabner J, Ramsey BW, Meeker DP, et al: Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J.Clin.Invest. 1996, 97: 1504-11.
Bellon G, Michel CL, Thouvenot D, et al: Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum.Gene Ther. 1997, 8: 15-25.
Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J: Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999, 274 (15): 10219-26. 10.1074/jbc.274.15.10219.
Pickles RJ, Fahrner JA, Petrella JM, Boucher RC, Bergelson JM: Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000, 74 (13): 6050-7. 10.1128/JVI.74.13.6050-6057.2000.
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR: Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993, 73: 309-19.
Grubb BR, Pickles RJ, Ye H, et al: Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994, 371: 802-6. 10.1038/371802a0.
Goldman M, Su Q, Wilson JM: Gradient of RGD-dependent entry of adenoviral vector in nasal and intrapulmonary epithelia: implications for gene therapy of cystic fibrosis. Gene Ther. 1996, 3: 811-8.
Goldman MJ, Wilson JM: Expression of alpha v beta 5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J.Virol. 1995, 69: 5951-8.
Pickles R, McCarty D, Matsui H, Hart PJ, Randell SH, Boucher RC: Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J.Virol. 1998, 72: 6014-23.
Engelhardt JF, Yankaskas JR, Ernst SA, et al: Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat.Genet. 1992, 2: 240-8.
Duan D, Sehgal A, Yao J, Engelhardt JF: Lef1 transcription factor expression defines airway progenitor cell targets for in utero gene therapy of submucosal gland in cystic fibrosis. Am.J.Respir.Cell Mol.Biol. 1998, 18: 750-8.
Pilewski JM, Engelhardt JF, Bavaria JE, Kaiser LR, Wilson JM, Albelda SM: Adenovirus-mediated gene transfer to human bronchial submucosal glands using xenografts. Am.J.Physiol. 1995, 268: L657-L665.
Griesenbach U, Chonn A, Cassady R, et al: Comparison between intratracheal and intravenous administration of liposome-DNA complexes for cystic fibrosis lung gene therapy. Gene Therapy. 1998, 5: 181-8. 10.1038/sj.gt.3300562.
Lemarchand P, Jones M, Danel C, Yamada I, Mastrangeli A, Crystal RG: In vivo adenovirus-mediated gene transfer to lungs via pulmonary artery. J Appl Physiol. 1994, 76 (6): 2840-5.
Charan NB, Carvalho PG: Anatomy of the normal bronchial circulatory systems in human and animals. The Bronchail Circulation. Lung Biol. Health Dis. Ser. Edited by: Butler J. 1992, New York: Dekker, 57: 45-77.
Magno M: Comparative anatomy of the tracheobronchial circulation. Eur Respir J Suppl. 1990, 12: 557s-563s.
Botenga AS: Selective Bronchial and Intercostal Arteriography. In: Williams & Wilkins, ed. Baltimore:. 1970, 1-159.
Kardjiev V, Symeonov A, Chankov I: Etiology, pathogenesis, and prevention of spinal cord lesions in selective angiography of the bronchial and intercostal arteries. Radiology. 1974, 112: 81-3.
Fellows KE, Khaw KT, Schuster S, Shwachman H: Bronchial artery embolization in cystic fibrosis; technique and long-term results. J.Pediatr. 1979, 95: 959-63.
Kohl T, Strumper D, Witteler R, Merschhoff G, Alexiene R, Callenbeck C, Asfour B, Reckers J, Aryee S, Vahlhaus C, Vogt J, Van Aken H, Scheld HH: Fetoscopic direct fetal cardiac access in sheep : An important experimental milestone along the route to human fetal cardiac intervention. Circulation. 2000, 102 (14): 1602-4.
Majno G, Palade GE: Studies on inflammation 1 The effect of histamine and serotonin on vascular permeability: An electron microscopic study. J Biophys Biochem Cytol. 1961, 11: 571-605.
Renkin EM, Carter RD, Joyner WL: Mechanism of the sustained action of histamine and bradykinin on transport of large molecules across capillary walls in the dog paw. Microvasc.Res. 1974, 7: 49-60.
Casley SJ, Window J: Quantitative morphological correlations of alterations in capillary permeability, following histamine and moderate burning, in the mouse diaphragm; and the effects of benzopyrones. Microvasc.Res. 1976, 11: 279-305.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983, 219: 983-5.
Neal CR, Michel CC: Transcellular gaps in microvascular walls of frog and rat when permeability is increased by perfusion with the ionophore A23187. J.Physiol.Lond. 1995, 488: 427-37.
Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am.J.Pathol. 1995, 146: 1029-39.
Isner JM, Pieczek A, Schainfeld R, et al: Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996, 348: 370-4. 10.1016/S0140-6736(96)03361-2.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2466/2/2/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Declaration of Competing Interests
We declare that we have participated in the construction of the manuscript titled "Bronchial Artery Delivery of Viral Vectors for Gene delivery in Cystic Fibrosis; Superior to Airway Delivery?" and that we have seen and approved the final version. We also declare that we have no conflict of interest in connection with this paper. Dr Ameet Bakhai MRCP Professor Desmond J. Sheridan FRCP Professor Charles C. Coutelle Dr.sc.med.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bakhai, A., Sheridan, D.J. & Coutelle, C.C. "Bronchial Artery Delivery of Viral Vectors for Gene delivery in Cystic Fibrosis; Superior to Airway Delivery?". BMC Pulm Med 2, 2 (2002). https://doi.org/10.1186/1471-2466-2-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2466-2-2