Abstract
Background
Recent studies have identified that a higher resting heart rate (RHR) is associated with elevated blood pressure, independent of body fatness, age and ethnicity. However, it is still unclear whether RHR can also be applied as a screening for other risk factors, such as hyperglycemia and dyslipidemia. Thus, the purpose of the presented study was to analyze the association between RHR, lipid profile and fasting glucose in obese children and adolescents.
Methods
The sample was composed of 180 obese children and adolescents, aged between 7-16 years. Whole-body and segmental body composition were estimated by Dual-energy X-ray absorptiometry. Resting heart rate (RHR) was measured by heart rate monitors. The fasting blood samples were analyzed for serum triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and glucose, using the colorimetric method.
Results
Fasting glucose, TC, triglycerides, HDL-C, LDL-C and RHR were similar in both genders. The group of obese subjects with a higher RHR presented, at a lower age, higher triglycerides and TC. There was a significant relationship between RHR, triglycerides and TC. In the multivariate model, triglycerides and TC maintained a significant relationship with RHR independent of age, gender, general and trunk adiposity. The ROC curve indicated that RHR has a high potential for screening elevated total cholesterol and triglycerides as well as dyslipidemia.
Conclusion
Elevated RHR has the potential to identify subjects at an increased risk of atherosclerosis development.
Similar content being viewed by others
Background
Over the last few decades obesity has reached epidemic proportions and become one of the major public health targets worldwide. Several researches indicate that obesity tracks from childhood to adulthood and constitutes a risk factor in the development of chronic diseases [1]. A high amount of body fatness is responsible for releasing a great amount of inflammatory adipokines into the bloodstream which has an important role in the pathogenesis of many chronic diseases [2, 3], and also in the changes of sympathetic and parasympathetic activity in children and adolescents, which can result in an increased resting heart rate (RHR) [4–7].
In adults, the use of RHR as screening index for cardiovascular risk has been postulated [8, 9] and supported by studies that reported its relationship to mortality, independent of abdominal obesity [10, 11], but few studies are found which focus on the obese pediatric population.
Recently, Fernandes et al. [6] identified that a higher RHR was associated with elevated blood pressure, in both lean and obese male children and adolescents, independent of age and ethnicity, however, it is not clear if RHR can also be applied as a screening for other risk factors, such as hyperglycemia and dyslipidemia.
Thus, the purpose of the present study was to analyze the association between RHR, lipid profile and fasting glucose in obese children and adolescents.
Methods
Sample
One hundred and eighty obese children and adolescents (97 male and 83 female), aged between 7-16 years, from Presidente Prudente, western Sao Paulo State, Brazil, were analyzed. The subjects were invited, through television and newspaper advertising, to participate in an intervention program, with physical activity and nutritional orientation, for obese boys and girls (in the present study only the initial data was used).
The participants were contacted initially by phone, after which an appointment was made in order to take measurements at the Campus of the Universidade Estadual Paulista - UNESP. Primary obesity diagnosis was made using body mass index (BMI) according to the cutoffs proposed by Cole et al. [12]. After the preliminary diagnosis of obesity, the following inclusion criteria were used to select the subjects: i) aged between six and 17 years; ii) no engagement in regular physical activity within the three months prior to the study; iii) no limitations on physical activity diagnosed by a medical doctor; iv) a consent form signed by parents/guardians to participate in the study. The present research was approved by the Ethical Research Expert Committee of the Universidade Estadual Paulista - Campus of Presidente Prudente (protocol number 087/2008).
Dual-energy X-ray absorptiometry (DEXA)
Whole-body and segmental body composition were estimated by Dual-energy X-ray absorptiometry (Lunar DPX-NT scanner [Lunar DPX-NT; General Electric Healthcare, Little Chalfont, Buckinghamshire, United Kingdom]) software version 4.7. Fat free mass (FFM), trunk fat mass (TFM) and percentage of body fatness (%BF) were measured. The DEXA and RHR measurements were made, on the same day, in a temperature-controlled room, in a laboratory at the University.
Resting heart rate
Portable heart rate monitors (S810; Polar Electro, Kempele, Finland) were used to measure RHR (expressed as beats per minute [beats/min]), which was monitored during two 30-second periods (with a three minute interval between them) in the sitting position. All measurements were registered after five minutes at rest in a quiet room with a constantly controlled temperature [8]. For statistical analysis, values of RHR were stratified into quartile: Quartile 1 (< 72 beats per minute [bpm]), Quartile 2 (72 - 78.4 bpm), Quartile 3 (78.5 - 84.9 bpm) and Quartile 4 (≥85 bpm).
Blood samples
Blood samples were collected with tubes containing EDTA and after a fasting night (10-12 hours). All collected blood samples (performed by nurses) and biochemical analyses were done in a private laboratory. The fasting blood samples were analyzed for serum triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and glucose, using the colorimetric method. Blood glucose ≥100 mg/dL was characterized as high blood glucose. Modifications in lipid profile were identified as: TC ≥170 mg/dL, LDL ≥130 mg/dL, HDL < 45 mg/dL or triglycerides ≥130 mg/dL [13]. The presence of, at least, one lipid modification was used to characterize the dyslipidemia diagnosis.
Pubertal stage
The stage of puberty was self-assessed by the participants. The subjects received a standardized series of drawings to assess their own pubertal development. Girls received drawings of the five stages of Tanner breast and female pubic hair development with appropriate descriptions accompanying the drawings. Boys received drawings showing the five Tanner stages of genitalia and pubic hair development, with appropriate written descriptions [14, 15]. The participants were asked to select the drawing of the stage that best indicated their own development (in cases where there was divergence between genitalia/breast stage and pubic hair stage [6% of the cases; n = 11], the genitalia/breast stage was adopted as pubertal stage). The results were placed by each subject in a locked box to guarantee the integrity and anonymity of the subjects, and only the main researcher had access to them.
Statistical Analysis
Mean and standard deviation were used as central tendency and dispersion measures, respectively. Students' tests and one-way analysis of variance followed by a Tukey's multiple comparison test were used in the comparisons among independent groups. The Pearson product-moment correlation coefficient was used to analyze the association between RHR and biochemical variables. In a multivariate regression model, all biochemical variables with p ≤ 0.20 were simultaneously inserted, which should explain which biochemical variables could be used as a function of RHR (expressed as beta values [β]; adjusted by age, gender, %BF, pubertal stage and TFM). The receiver-operating characteristic (ROC) curve is a valuable tool for the assessment of the accuracy of diagnostic tests and provides a powerful means with which to assess the test's ability to discriminate between the true-positive ratio (sensitivity) and the true-negative ratio (specificity) [16]. For categorical analyses, the chi-square test (χ2) was used to determine the existence of a significant association between RHR quartiles and dyslipidemia. Statistical significance was set at < 5% and statistical software SPSS version 13.0 (SPSS Inc, Chicago, Illinois) was used for all analyses.
Results
Table 1 shows the general characteristics of the sample stratified by sex. There was an average age of 11.2 ± 2.7 years, which was similar in both sexes. The males were taller, heavier and presented a higher amount of trunk fat. Comparisons of the %BF, between the sexes, were on the borderline of statistical significance. Fasting glucose (p = 0.064), TC (p = 0.640), triglycerides (p = 0.254), HDL-C (p = 0.271), LDL-C (p = 0.637) and RHR (p = 0.169) were similar in both sexes. Pubertal stages were similar in both boys and girls.
Table 2 presents values of age and biochemical analysis distributed per quartile of RHR. The group of obese subjects with a higher RHR presented lower ages, higher triglycerides and TC. There was a similarity in trunk fat, fasting glucose, HDL-C, %BF and LDL-C.
A statistical relationship was observed between RHR and triglycerides and RHR and TC, but not between RHR and LDL-C (Table 3). In the multivariate model, only triglycerides and TC maintained a significant relationship with RHR, independent of age, pubertal stage, sex, general and trunk adiposity. There was a significant relationship between pubertal stage and TC (r = -0.15; p = 0.046), %BF (r = 0.19; p = 0.012), TFM (r = 0.60; p = 0.001) and RHR (r = -0.26; p = 0.001); but not for HDL-C, LDL-C, fasting glucose and triglycerides.
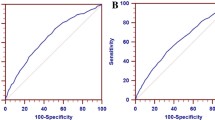
The ROC curve indicated that RHR has limited potential for screening elevated LDL-C (AUC: 0.584 ± 0.044; p = 0.052), but high potential for screening elevated total cholesterol (AUC: 0.609 ± 0.042; p = 0.014) and triglycerides (AUC: 0.650 ± 0.042; p = 0.001), as well as dyslipidemia (AUC: 0.658 ± 0.052; p = 0.010) (Figure 1). Finally, the number of modifications in lipid profile was inversely associated with RHR quartile (p = 0.001; Figure 2, Panel A) and the occurrence of dyslipidemia was higher in the higher quartile for resting HR (p = 0.027; Figure 2, Panel B).
Discussion
The present study was carried out on obese children and adolescents, of both sexes, and identified that RHR has a significant relationship to dyslipidemia.
Previous studies have identified that, in children and adolescents, the chronological age is inversely related to RHR [6, 17]. Al-Qurashi et al. [5] proposed age-specific reference values of RHR to Saudi children/adolescents, and identified that the RHR values were lower in adolescents than in children. A possible explanation for this is the alteration in the autonomic cardiac control, which is age dependent.
Previous studies carried out on subjects from birth to 24 years observed changes in the autonomic nervous system in accordance with nutritional status and advancing age [18, 19]. They observed that sympathetic and parasympathetic activity increase in infants but, in children and adolescents, there is a great decrease in sympathetic activity and only a slight decrease in parasympathetic activity. Therefore, the lower cardiac sympathetic activity in children and adolescents may explain the reduction in the RHR values [5, 6, 17] observed in the present study, and support the necessity to adjust the statistical analyzes by age.
In this obese sample, both elevated occurrences of dyslipidemia (85.6%) and elevated blood pressure (17.8% [data not shown]) were observed. In fact, scientific literature has linked dyslipidemia and arterial hypertension to increased adiposity in children and adolescents [6, 20, 21]. In pediatric obesity, the endothelial dysfunction occurs due to a state of increased oxidative stress and the action of the vascular cells adhesion molecules [20, 21]. Moreover, the above mentioned inflammatory mechanisms are strongly related to dyslipidemia [22]. Our data agrees with previous research, in which there is an elevated occurrence of the components of metabolic syndrome in obese Brazilian youths [23]. Caranti et al. [23] identified that metabolic syndrome has a higher occurrence in obese Brazilian youths (34.8% in boys and 15.6% in girls) than in obese Italian youths (23.6% in boys and 12.5% in girls). The above mentioned data reinforces the dramatic necessity to implement effective public health action, targeting the prevention of pediatric obesity in developing nations.
It is well established that the practice of regular physical activity improves the production of superoxide dismutase and nitric oxide [24, 25] and, in turn, that regular physical activity from an early age, prevents the development of cardio-metabolic and cardiovascular diseases in adulthood [26]. In the present study, the sedentarism of the sample participants could be a factor in justifying the elevated occurrence of dyslipidemia and elevated blood pressure.
Research has shown an increased sympathetic activity in obese individuals [27–29]. Similarly, even in healthy normal weight subjects, the venous infusion of non-esterified fatty acids increases central sympathetic activation [30], while weight loss decreases sympathetic activity [31, 32]. Our findings indicate the potential of RHR to screen dyslipidemia in obese children and adolescents. On the other hand, the observed relationship between tachycardia and dyslipidemia is not as simple to explain, because it is affected by many pathways and the causality in these biological mechanisms is still not clear [2].
The actual function of some adipokines that affect the insulin binding by blocking the insulin receptor substrates-1 activation, stimulate the lipolysis and contribute to development of dyslipidemia, was recently described [2]. These adipokines increase the production of reactive oxygen species in the brain, through activation of the nicotine adenine dinucleotide hydrogen phosphatase oxidase, increasing the oxidative stress in rostral ventrolateral medulla, which determinates the basal sympathetic activity [33, 34]. In fact, recent studies have reported that the status of oxidative stress affects, positively, the sympathetic nervous system activation, which is responsible for the increase of RHR [33, 34].
In our study, fasting glucose was not related to RHR. Oda and Kawai [10] identified, in a large sample of Japanese adults, increased fasting glucose in subjects with a higher RHR. Likewise, our results do not support these results, because our sample was composed exclusively of obese children and adolescents and further studies are necessary to clarify this issue.
A positive aspect of the present study is the analysis of TFM by DEXA. The inclusion of TFM in the multivariate model was important because this adipose tissue is related to the increased release of adipokines related to many pro-inflammatory mechanisms [2, 3]. Moreover, our data indicated an important relationship between sexual maturity and higher TC, lower RHR and higher body fatness and, therefore, to take into account the pubertal stage in the analysis (instead of only chronological age) makes the findings more consistent, because sexual maturity is strongly related to factors that directly affect the RHR and lipid profile (e.g. hypertrophy/hyperplasia of adipose tissue, increased release of hormones and adipokines) [35].
On the other hand, some limitations must be pointed out. The cross-sectional design does not offer support to causality statements and, therefore, prospective studies from childhood to adolescence are necessary to describe more accurately the longitudinal relationship between RHR and dyslipidemia. The absence of inflammatory markers related to oxidative stress and, the absence of insulin measures to screen more clearly the relationship between RHR and glucose metabolism should be considered in future research.
Conclusions
In summary, we conclude that increased RHR was significantly associated with dyslipidemia in obese children and adolescents and that elevated RHR offers potential to screen subjects at an increased risk of atherosclerosis development. However, longitudinal and epidemiological surveys should be carried out to develop optimal cutoff values for RHR in pediatric populations.
Contribution of the Authors
RAF: (1) conception and design of the study, (2) acquisition, analysis and interpretation of data, (3) draft of the article and selection of manuscripts to discuss the results, PAM, LSS, SAU, BMA, KNB and JSC: (1) Acquisition, analysis and interpretation of data, (2) draft of the article and selection of manuscripts to discuss the results, IFFJ and JPJS: (1) conception and design of the study (2) review and approval of the final version to be submitted. All authors read and approved the final manuscript.
Abbreviations
- RHR:
-
resting heart rate
- BMI:
-
body mass index
- DEXA:
-
dual-energy X-ray absortometry
- FFM:
-
fat free mass
- TFM:
-
trunk fat mass
- %BF:
-
body fat percentage
- Beats/min:
-
beats per minute
- TC:
-
total cholesterol
- HDL-C:
-
high-density lipoprotein cholesterol
- LDL-C:
-
low-density lipoprotein cholesterol
- TC:
-
total cholesterol
- ROC:
-
receiver operation characteristic
- SD:
-
standard-deviation.
References
Sinaiko AR, Donahue RP, Jacobs DR, Prineas RJ: Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999, 99: 1471-6.
Huang PL: eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009, 20: 295-302. 10.1016/j.tem.2009.03.005.
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G: Mechanisms of obesity-induced hypertension. Hypertens Res. 2010, 33: 386-93. 10.1038/hr.2010.9.
Rabbia F, Grosso T, Cat Genova G, Conterno A, De Vito B, Mulatero P, Chiandussi L, Veglio F: Assessing resting heart rate in adolescents: determinants and correlates. J Hum Hypertens. 2002, 16: 327-32. 10.1038/sj.jhh.1001398.
Al-Qurashi MM, El-Mouzan MI, Al-Herbish AS, Al-Salloum AA, Al-Omar AA: Age related reference ranges of heart rate for Saudi children and adolescents. Saudi Med J. 2009, 30: 926-31.
Fernandes RA, Freitas Junior IF, Codogno JS, Christofaro DG, Monteiro LH, Lopes DM: Resting hearth rate is associated with blood pressure in male children and adolescents. J Pediatr. 2011, 158: 634-7. 10.1016/j.jpeds.2010.10.007.
Baba R, Koketsu M, Nagashima M, Inasaka H, Yoshinaga M, Yokota M: Adolescent obesity adversely affects blood pressure and resting heart rate. Circ J. 2007, 71: 722-726. 10.1253/circj.71.722.
Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Pessina AC, Ruilope LM, Zanchetti A, European Society of Hypertension: Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens. 2006, 24: 603-10. 10.1097/01.hjh.0000217838.49842.1e.
Palatini P: Elevated heart rate: a "new" cardiovascular risk factor?. Prog Cardiovasc Dis. 2009, 52: 1-5. 10.1016/j.pcad.2009.06.001.
Oda E, Kawai R: Significance of heart rate in the prevalence of metabolic syndrome and its related risk factors in Japanese. Circ J. 2009, 73: 1431-6. 10.1253/circj.CJ-08-1142.
Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM: Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010, 159: 612-619. 10.1016/j.ahj.2009.12.029.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH: Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000, 320: 1-6. 10.1136/bmj.320.7226.1.
Brazilian Society of Cardiology: I Guideline of Prevention of atherosclerosis in childhood and adolescence. Arq Bras Cardiol. 2005, 85: 1s-36s.
Marshall WA, Tanner JM: Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969, 44: 291-303. 10.1136/adc.44.235.291.
Marshall WA, Tanner JM: Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970, 45: 13-23. 10.1136/adc.45.239.13.
Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, Haghazali M, Asgari F: Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond). 2010, 7: 26-10.1186/1743-7075-7-26.
Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, et al: Assesssment of cardiac autonomic modulation during adolescente obesity. Obesity research. 2003, 11: 541-8. 10.1038/oby.2003.76.
Finley JP, Nugent ST, Hellenbrand W: Heart-rate variability in children. Spectral analysis of developmental changes between 5 and 24 years. Can J Physiol Pharmacol. 1987, 65: 2048-52. 10.1139/y87-320.
Finley JP, Nugent ST: Heart rate variability in infants, children and young adults. J Auton Nerv Syst. 1995, 2: 103-8.
Kelly AS, Hebbel RP, Solovey AN, Schwarzenberg SJ, Metzig AM, Moran A, Sinaiko AR, Jacobs DR, Steinberger J: Circulation activated endothelial cells in pediatric obesity. J Pediatr. 2010, 157: 547-51. 10.1016/j.jpeds.2010.04.069.
Ostrow V, Wu S, Aguilar A, Bonner R, Suarez E, de Luca F: Association between oxidative stress and masked hypertension in a multi-ethnic population of obese children and adolescents. J Pediatr. 2011, 158: 628-33. 10.1016/j.jpeds.2010.09.081.
Diaz MN, Frei B, Vita JA, Keaney JF: Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997, 337: 408-16. 10.1056/NEJM199708073370607.
Caranti DA, Lazzer S, Dâmaso AR, Agosti F, Zennaro R, de Mello MT, Tufik S, Sartorio A: Prevalence and risk factors of metabolic syndrome in Brazilian and Italian obese adolescents: a comparison study. Int J Clin Pract. 2008, 62: 1526-32. 10.1111/j.1742-1241.2008.01826.x.
de Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A: Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol. 2008, 8: 12-10.1186/1472-6793-8-12.
Zaros PR, Pires CE, Bacci M, Moraes C, Zanesco A: Effect of 6-months of physical exercise on the nitrate/nitrite levels in hypertensive postmenopausal women. BMC Womens Health. 2009, 9: 17-10.1186/1472-6874-9-17.
Fernandes RA, Zanesco A: Early physical activity promotes lower prevalence of chronic diseases in adulthood. Hypertens Res. 2010, 33: 926-31. 10.1038/hr.2010.106.
Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G: Sympathetic activation in obese normotensive subjects. Hypertension. 1995, 25: 560-3.
Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG: Overweight and sympathetic overactivity in black Americans. Hypertension. 2001, 38: 379-83.
Alvarez GE, Beske SD, Ballard TP, Davy KP: Sympathetic neural activation in visceral obesity. Circulation. 2002, 106: 2533-6. 10.1161/01.CIR.0000041244.79165.25.
Florian JP, Pawelczyk JA: Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin Sci. 2010, 118: 61-9.
Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G: Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998, 97: 2037-42.
Trombetta IC, Batalha LT, Rondon MU, et al: Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003, 285: H974-82.
Hirooka Y, Sagara Y, Kishi T, Sunagawa K: Oxidative stress and central cardiovascular regulation. Circ J. 2010, 74: 827-35. 10.1253/circj.CJ-10-0153.
Hirooka Y: Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hpertens Res. 2011, 34: 407-12. 10.1038/hr.2011.14.
Malina RM, Bouchard C, Bar-Or O: Growth, Maturation, and Physical Activity. 2004, Champaign: Human Kinetics, 2
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/12/5/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Freitas Júnior, I.F., Monteiro, P.A., Silveira, L.S. et al. Resting heart rate as a predictor of metabolic dysfunctions in obese children and adolescents. BMC Pediatr 12, 5 (2012). https://doi.org/10.1186/1471-2431-12-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-12-5