Abstract
Background
Cancer of the esophagus is a deadly malignancy, and development of biomarkers that predict survival is an urgent need. The apoptotic pathways have been hypothesized as important in progression of esophageal squamous cell carcinoma (ESCC). We investigated a panel of proteins that regulate apoptosis as candidate of biomarkers of prognosis in ESCC.
Methods
Tissue microarray (TMA) including 313 surgically-resected cases of ESCC specimens was built for immunohistochemical interrogation. We evaluated seven genes in the FasL-Fas apoptotic pathway - FasL, Fas, FAS-associated death domain protein (FADD), phosphorylated-FADD, and caspase 8 and 10, and the antiapoptotic protein bcl-2. We studied pathway integrity and relations to risk and clinical factors, and determined the prognostic significance of each marker.
Results
Five markers showed strong inter-marker correlations (r ≥ 0.28, p < 0.001), including FasL, Fas, FADD, and caspases 8 and 10. FasL and FADD also showed modest correlations with one or more cancer risk factors, but none of the markers was significantly associated with either tumor stage or lymph node metastasis, the only two clinical factors that predicted survival in these ESCC cases. Multivariate-adjusted proportional hazard regression models showed no association between protein expression and risk of death for any of the seven markers examined.
Conclusion
Individual biomarkers in the apoptosis pathway do not appear to predict survival of patients with ESCC.
Similar content being viewed by others
Background
Fas-mediated apoptosis is thought to be involved in the initiation and development of esophageal squamous cell carcinoma (ESCC). Previous gene expression profiling of ESCC showed over-expression of FAS-associated death domain RNA (FADD) and under-expression of Fas and caspase 8 [1]. The phosphorylated form of FADD (p-FADD) has recently been reported to regulate apoptotic activity [2]. Although the role of p-FADD in ESCC outcome is unclear, higher levels of p-FADD protein correlated with reduced survival in patients with lung adenocarcinomas [3] and prostate cancer [4].
Using an ESCC tissue microarray (TMA) [5], we explored the expression of FasL, Fas, FADD, p-FADD, caspase 8 and 10, which are proteins involved in the FasL-Fas apoptotic pathway, and the antiapoptotic protein bcl-2. We determined the prevalence of protein expression for each marker, investigated pathway integrity by evaluating the correlations between individual markers as well as between markers and risk factors/clinico-pathologic features, and we examined the prognostic significance of the markers on the survival of ESCC cases.
Methods
Patient selection
This study was approved by the Institutional Review Boards of the Shanxi Cancer Hospital and the U.S. National Cancer Institute. Patients presenting to the Shanxi Cancer Hospital in Taiyuan, Shanxi, People's Republic of China between 1996 and 2001 were eligible for inclusion in this study. The Shanxi Cancer Hospital, the largest cancer hospital in Shanxi, performed surgery on approximately 2000 new esophageal annually during the study period. We included cases in this study who: (i) were males or females 20 years of age or older, (ii) had newly diagnosed (incident) cancer of the esophagus without previous treatment (including surgery, chemotherapy, or radiotherapy), (iii) underwent surgical resection of their tumor at the Shanxi Cancer Hospital, and (iv) had their diagnosis histologically confirmed. Since a primary objective of this study was to evaluate somatic changes in tumors, we limited recruitment to patients who had complete surgical resection of their tumor as their primary therapy; approximately 50% of new ESCC cases underwent surgical resection as their primary therapy. Neoadjuvant and adjuvant therapy were not employed at the Shanxi Cancer Hospital in surgically resected ESCC cases during the time period that this study was conducted. Esophageal cancer cases were limited to those with histological ESCC, which included nearly all esophageal cancers since adenocarcinoma of the esophagus is essentially nonexistent in this high-risk population. All histological diagnoses were made initially by pathologists at the Shanxi Cancer Hospital and confirmed by pathologists at the National Cancer Institute. In addition to confirmation of their histologic diagnosis, cases were classified as either well differentiated or poorly differentiated ESCC.
We collected information on demographic and lifestyle cancer risk factors [eg, smoking, alcohol drinking, family history of upper gastrointestinal (UGI) cancer] on cases using a structured interview with a questionnaire administered by a nurse in the hospital prior to surgery. Clinical data was abstracted from hospital records after surgery. Demographic, lifestyle and clinical data for cases included in this study are shown in Table 1. All patients (or their family members) were re-contacted in 2003 to ascertain vital status.
Tissue microarray (TMA) construction
Details of the TMA construction were previously described [5]. In brief, the TMA was constructed with surgical resection tissue samples from 313 ESCC cases, and selected control tissues using 0.6 mm needles. After exclusion of cores with inadequate tissue following sectioning and tissue transfer, the final immunohistochemical analyses included cores from 265 ESCC cases. Each of the 265 different ESCC cases contributed to one or more of the different biomarker analyses. Final numbers of cases for each of the biomarkers evaluated here are shown in Table 2.
Immunohistochemistry staining and assessment
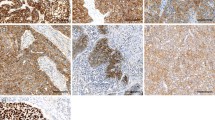
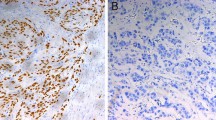
The TMA sections were stained with antibodies to FasL (Lab Vision Corp., CA), Fas (Santa Cruz, CA), FADD (Novocastra, UK), p-FADD (Cell Signaling Technology, MA), caspase 8 (Lab Vision Corp., CA), caspase 10 (Cell Signaling Technology, MA), and bcl-2 (Dako, CA). Slides were stained according to manufacturer's protocols. The immunohistochemical staining patterns were validated against previously described patterns of staining for each marker on a separate TMA, including appropriate positive and negative controls. Internal positive and negative controls, including normal squamous epithelium of the esophagus from non-cancer patients were utilized as available to further support the staining patterns.
Fas and FasL was expressed exclusively in cell membranes, whereas immunoreactivity of FADD and bcl-2 was cytoplasmic. Caspase 8 and 10 expression was detected in both cytoplasm and nucleus. p-FADD was primarily expressed in the nucleus of cells. Staining results were scored based on: (i) percent of positive tumor cells in tumor tissue: zero (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%); and (ii) signal intensity: zero (no signal), 1 (weak), 2 (moderate) and 3 (marked). An overall score was calculated by multiplying the percent cells positive score by the intensity score (range zero to 12).
Statistical analysis
All statistical analyses were performed using Statistical Analysis Systems (SAS) (SAS Corp, NC). Spearman correlation coefficients were used to assess associations between the seven different apoptosis biomarkers, and between the seven markers and risk factors/clinico-pathologic features. Overall survival time was calculated as the date of surgery to the date of death or the date last known alive. Survival was examined graphically with Kaplan-Meier curves and analyzed statistically with log-rank tests and proportional hazards regression models (SAS PHREG procedure) adjusted for lifestyle and tumor characteristics as covariates as previously described [6]. All P-values were two-sided and considered statistically significant if P < 0.05.
Results
Overall protein expression for the seven markers evaluated here showed low levels of positivity, ranging from zero to 15% positive (Table 2). The Spearman correlation coefficients revealed that expression of FasL and Fas, FasL and caspase 10, FADD and caspase 8, FADD and caspase 10, and caspases 8 and 10 in ESCC were strongly associated (r ≥ 0.28, p < 0.0001). Fas and caspase 8, and Fas and caspase 10 were also significantly correlated (r = 0.18, p < 0.01). Moreover, FasL and FADD, FasL and caspase 8, and caspase 10 and bcl-2 were mildly associated (r = 0.15 to 0.16, p < 0.05) (Table 3).
We successfully contacted 261 of 265 ESCC cases or their families during follow-up for vital status; 181 cases died and 80 were still alive at the end of follow-up. Median overall survival was 677 days (one year, 10 months) and 15 cases were still alive five or more years post-surgery; the longest survivor was still alive six years and two months after surgery. Kaplan-Meier graphs and log-rank analyses of individual markers did not reveal differences in overall survival by protein expression positivity (data not shown). Analyses adjusted for demographic attributes and potential confounding factors in Cox proportional hazard regression models also failed to identify significant associations between markers and survival time (Table 2).
Table 4 shows the Spearman correlations between the seven apoptosis biomarkers and five risk factors, which include sex, age, tobacco use, alcohol use and family history of upper gastrointestinal cancer. Significant associations were observed between FasL expression and male sex (r = 0.17), tobacco use (r = 0.17), and family history of UGI cancer (r = -0.18) (all p values < 0.01). Mild correlation was seen between caspase 8 expression and alcohol use, but it did not reach statistical significance. The Spearman correlation between the seven apoptosis biomarkers and four clinico-pathologic features were also studied (Table 4). Histological differentiation was strongly correlated with Fas, caspase 10, and bcl-2 expressions (r = -0.26 to -0.36, all p values < 0.0001); lower (but still statistically significant) correlations were observed with FADD, caspase 8, and FasL. In addition, significant associations were also seen for tumor grade with Fas, FADD, and caspase 10, and for tumor stage with Fas expression.
Relations to survival adjusted for these five risk factors and four clinico-pathologic features are shown in Table 5. Higher tumor stage [hazard ratio (HR), 1.92; 95% confidence interval (95% CI), 1.14-3.23] and presence of lymph node metastasis (HR, 2.17; 95% CI 1.59-2.95) were significantly and independently associated with death.
Discussion
The results of correlation analyses confirmed that these biomarkers in the FasL-Fas apoptotic pathway were closely related. The expression of p-FADD was generally low in this study, and it may explain why we did not detect correlations between p-FADD and other markers. Bcl-2 inhibits BAX (Bcl-2-associated X protein)/BAK (Bcl-2-antagonist/killer1) proteins, which induce the permeabilization of the outer mitochondrial membrane, a crucial step for apoptotic cell death [7]. Caspase 8 is involved in functions related to bcl-2 and BAX/BAK proteins. Bcl-2 was not significantly correlated with caspase 8, but was mildly associated with caspase 10. Although caspase 10 does not seem to directly interact with bcl-2 in the apoptosis pathway, correlation of expression may be anticipated, given the complexity of apoptosis regulation.
In accord with our findings, Xue et al. also previously reported that Fas and FasL were not related to disease-free survival in ESCC [8]. In contrast, Kase et al. observed significantly longer ESCC-free survival in patients with Fas-positive (versus Fas-negative) tumors, and in FasL-negative (versus FasL-positive) tumors [9]. Shibakita et al. reported that Fas expression was an independent prognosticator for recurrence-free survival, but that FasL expression did not influence ESCC survival.[9] Studies of the prognostic significance of caspase 8 are limited to a single previous report in which no effect on survival was noted [10]. There are no published studies thus far on the prognostic significance of caspase 10 in ESCC.
FADD did not predict survival in ESCC in our study, a finding similar to that of Chang et al [11]. In contrast, Xue et al. reported that FADD expression correlated with decreased survival in ESCC [8]. Induction of p-FADD results in suppression of cancer cell growth and invasion through reduction in the non-phosphorylated form in prostate cancer [12]. Consistent with two previous reports, we also were unable to relate bcl-2 expression to prognosis [13, 14]. Chang et al. found that bcl-2 expression correlated with better survival, and was an independent prognostic factor after multivariate analysis[11]. Similar findings were noted by Parenti et al. and Ohbu et al., however, in both studies bcl-2 was not an independent prognostic value after adjustment for other variables in multivariate analysis [15, 16].
FasL expression showed significant correlation with three risk factors including male sex, tobacco use, and family history of UGI cancer (Table 4). Fas had mild association with family history of UGI cancer. Interaction of Fas and FasL initiates Fas-mediated apoptosis and transmits signals to the downstream of the pathway. It suggests that these risk factors might closely relate to Fas-mediated apoptosis pathway and contribute to the pathogenesis of ESCC. Among the clinico-pathologic features we studied (Table 4), tumor grade was significantly associated with Fas, FADD and caspase 10. Histological differentiation of ESCC was significantly correlated with the target biomarkers except p-FADD, which exhibited very low expression. In the present study, we used a TMA to analyze a larger number of ESCC cases for apoptotic pathway markers than any previous report in the literature. Further, performance of IHC on a single slide under identical conditions should minimize variability in staining and thus enhance the reliability of our results. Despite the size and other favorable characteristics of the current study, the prevalence of protein positivity for the apoptosis pathway markers examined was low (≤ 15%) and the differences in survival between protein expression positive versus negative groups were small, resulting in only limited power (<10%) to distinguish the small differences in survival actually observed between groups here. For the markers with the highest positivity (ie, 15%) in this study, we had good (ie, 80%) power to detect only much larger hazard ratios that were observed here (ie, 3.4 or greater).
Conclusion
While the current study evaluated only protein expression in relation to survival and not the potential use of these biomarkers in the early detection of ESCC, the generally low prevalence of expression positivity indicates that they would not be suitable candidates for early detection markers. We were unable to identify a role for biomarkers in the FasL-Fas apoptotic pathway and prognosis in ESCC.
References
Su H, Hu N, Shih J, Hu Y, Wang Q, Chuang E, Roth M, Wang C, Goldstein A, Ding T, et al: Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res. 2003, 63 (14): 3872-3876.
Screaton R, Kiessling S, Sansom O, Millar C, Maddison K, Bird A, Clarke A, Frisch S: Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proc Natl Acad Sci USA. 2003, 100 (9): 5211-5216. 10.1073/pnas.0431215100.
Chen G, Bhojani M, Heaford A, Chang D, Laxman B, Thomas D, Griffin L, Yu J, Coppola J, Giordano T, et al: Phosphorylated FADD induces NF-kappaB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. Proc Natl Acad Sci USA. 2005, 102 (35): 12507-12512. 10.1073/pnas.0500397102.
Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Konishi N: Phosphorylation status of Fas-associated death domain-containing protein (FADD) is associated with prostate cancer progression. J Pathol. 2005, 206 (4): 423-432. 10.1002/path.1791.
Shou J, Hu N, Takikita M, Roth M, Johnson L, Giffen C, Wang Q, Wang C, Wang Y, Su H, et al: Overexpression of CDC25B and LAMC2 mRNA and protein in esophageal squamous cell carcinomas and premalignant lesions in subjects from a high-risk population in China. Cancer Epidemiol Biomarkers Prev. 2008, 17 (6): 1424-1435. 10.1158/1055-9965.EPI-06-0666.
Hu N, Flaig M, Su H, Shou J, Roth M, Li W, Wang C, Goldstein A, Li G, Emmert-Buck M, et al: Comprehensive characterization of annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res. 2004, 10 (18 Pt 1): 6013-6022. 10.1158/1078-0432.CCR-04-0317.
Youle R, Strasser A: The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008, 9 (1): 47-59. 10.1038/nrm2308.
Xue L, Ren L, Luo W, Guan X, Zou S, Zheng S, Bi R, Xie Y, He Z, Lü N: [Expression of Fas, Fas ligand, Fas-associated death domain protein, caspase 8 and mutant P53 protein in esophageal squamous cell carcinoma]. Zhonghua Yi Xue Za Zhi. 2007, 87 (3): 150-154.
Kase S, Osaki M, Adachi H, Kaibara N, Ito H: Expression of Fas and Fas ligand in esophageal tissue mucosa and carcinomas. Int J Oncol. 2002, 20 (2): 291-297.
Shibakita M, Tachibana M, Dhar D, Kotoh T, Kinugasa S, Kubota H, Masunaga R, Nagasue N: Prognostic significance of Fas and Fas ligand expressions in human esophageal cancer. Clin Cancer Res. 1999, 5 (9): 2464-2469.
Chang M, Lee H, Lee B, Kim Y, Lee J, Kim W: Differential protein expression between esophageal squamous cell carcinoma and dysplasia, and prognostic significance of protein markers. Pathol Res Pract. 2005, 201 (6): 417-425. 10.1016/j.prp.2005.04.005.
Shimada K, Nakamura M, Ishida E, Konishi N: Molecular roles of MAP kinases and FADD phosphorylation in prostate cancer. Histol Histopathol. 2006, 21 (4): 415-422.
Takayama T, Nagao M, Sawada H, Yamada Y, Emoto K, Fujimoto H, Ueno M, Hirao S, Nakajima Y: Bcl-X expression in esophageal squamous cell carcinoma: association with tumor progression and prognosis. J Surg Oncol. 2001, 78 (2): 116-123. 10.1002/jso.1130.
Sarbia M, Bittinger F, Porschen R, Verreet P, Dutkowski P, Willers R, Gabbert H: bcl-2 expression and prognosis in squamous-cell carcinomas of the esophagus. Int J Cancer. 1996, 69 (4): 324-328. 10.1002/(SICI)1097-0215(19960822)69:4<324::AID-IJC15>3.0.CO;2-5.
Parenti A, Rugge M, Shiao Y, Ruol A, Ancona E, Bozzola L, Ninfo V: bcl-2 and p53 immunophenotypes in pre-invasive, early and advanced oesophageal squamous cancer. Histopathology. 1997, 31 (5): 430-435. 10.1046/j.1365-2559.1997.2970888.x.
Ohbu M, Saegusa M, Kobayashi N, Tsukamoto H, Mieno H, Kakita A, Okayasu I: Expression of bcl-2 protein in esophageal squamous cell carcinomas and its association with lymph node metastasis. Cancer. 1997, 79 (7): 1287-1293. 10.1002/(SICI)1097-0142(19970401)79:7<1287::AID-CNCR3>3.0.CO;2-E.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/9/310/prepub
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and Division of Cancer Epidemiology and Genetics.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NH, PRT, and SMH designed the study. NH, JZS, and QHW were responsible for management of patients' data and tissues. MT and SMH performed IHC and provided the scoring data. CG, NH, and PRT performed statistical analysis. MT and SMH wrote the manuscript, and NH and PRT helped editing the manuscript. All authors read and approved the final version of the manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Takikita, M., Hu, N., Shou, Jz. et al. Biomarkers of apoptosis and survival in esophageal squamous cell carcinoma. BMC Cancer 9, 310 (2009). https://doi.org/10.1186/1471-2407-9-310
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-9-310