Abstract
Background
Our previous studies showed that ZBTB20, a new BTB/POZ-domain gene, could negatively regulate α feto-protein and other liver-specific genes, concerning such as bio-transformation, glucose metabolism and the regulation of the somatotropic hormonal axis. The aim of this study is to determine the potential clinical implications of ZBTB20 in hepatocellular carcinoma (HCC).
Methods
Quantitative real-time RT-PCR and Western blot analyses were used to detect expression levels of ZBTB20 in 50 paired HCC tumorous and nontumorous tissues and in 20 normal liver tissues. Moreover, expression of ZBTB20 was assessed by immunohistochemistry of paired tumor and peritumoral liver tissue from 102 patients who had undergone hepatectomy for histologically proven HCC. And its relationship with clinicopathological parameters and prognosis was investigated.
Results
Both messenger RNA and protein expression levels of ZBTB20 were elevated significantly in HCC tissues compared with the paired non-tumor tissues and normal liver tissues. Overexpressed ZBTB20 protein in HCC was significantly associated with vein invasion (P = 0.016). Importantly, the recurrence or metastasis rates of HCCs with higher ZBTB20 expression were markedly greater than those of HCCs with lower expression (P = 0.003, P = 0.00015, respectively). Univariate and multivariate analyses revealed that ZBTB20 overexpression was an independent prognostic factor for HCC. The disease-free survival period and over-all survival period in patients with overexpressed ZBTB20 in HCC was significantly reduced.
Conclusions
The expression of ZBTB20 is increased in HCC and associated with poor prognosis in patients with HCC, implicating ZBTB20 as a candidate prognostic marker in HCC.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the fifth most-common malignancy in the world and is the third cause of cancer-related death worldwide. The development and progression of HCC is a complicated process involving multiple genes and several transforming steps [1, 2]. The exact molecular mechanisms underlying hepatocarcinogenesis have not yet been elucidated. Therefore, searching for new HCC associated molecules may give some clues to study the mechanism of HCC and provide prognostic value in clinical issues.
The BTB/POZ-ZF [Broad complex, Tramtrack, Bric a' brac (BTB) or poxvirus and zinc finger (POZ)-zinc finger] protein family comprises a diverse group of transcription factors. These factors are so named because of a distinct and unique N-terminal BTB/POZ domain and C-terminal DNA-binding zinc finger domains. These proteins have been demonstrated to participate in a wide variety of cellular functions including transcriptional regulation, cellular proliferation, apoptosis, cell morphogenesis, ion channel assembly, and protein degradation through ubiquitination-proteasome system [3–5]. A subset of BTB/POZ proteins have been implicated in human cancer, and they include BCL-6 (B-cell lymphoma 6) [6–9], PLZF (promyelocytic leukemia zinc finger) [10–14], leukemia/lymphoma-related factor (LRF)/Pokemon [15–18], HIC-1 (hypermethylated in cancer-1) [19–23], NAC-1[24–29] and Kaiso [30–33].
ZBTB20 gene, also named DPZF [34], HOF [35], and ZNF288 [36], is a new member of the BTB/POZ-ZF family. It has two isoforms due to the alternative translation initiation, both containing an intact N-terminal BTB domain and a C-terminal zinc finger domain [35]. Zbtb20 is preferentially expressed by hippocampal progenitors, and essential for hippocampal development [37].
In liver, our previous work showed that human ZBTB20 is expressed in fetal liver [34]. In mouse, Zbtb20 is developmentally up-regulated in postnatal liver, and acts as a key transcription repressor of AFP [38]. What's more, ZBTB20 may play an essential role in liver intrinsic functions, possibly through regulating genes such as P450 family members, glucose metabolism and the regulation of the somatotropic hormonal axis [39].
In the present study, we investigated the expression of ZBTB20 in HCC tissues and its potential association with clinicopathological features and post-resectional survival. Our results suggested that ZBTB20 overexpression can be used as an independent marker for the prognosis of patients with HCC.
Methods
Patients and liver specimens
Primary HCC tissue sample (n = 152) were randomly obtained from patients who underwent routine curative surgery at the Eastern Hepatobiliary Surgery Hospital between 2001 and 2007. The patients were not pretreated with radiotherapy or chemotherapy prior to surgery. Among them, 102 tissue samples were fixed in 10% formalin and embedded in paraffin for immunohistochemical analysis. The other 50 tissue samples were immediately frozen in liquid nitrogen and stored at -80°C after hepatectomy for western blotting and real-time RT-PCR. Table 1 shows the clinicopathological features of these patients. Additionally, normal liver specimens were obtained from 20 patients with hemangiomas of liver who underwent surgery. The 20 cirrhotic liver specimens were obtained from patients who underwent liver biopsy. The diagnoses were confirmed by histopathologic study. Tumor stage was determined according to the 2002 International Union Against Cancer TNM classification system [40]. Tumor differentiation was graded by the Edmondson grading system. Written informed consent was obtained from these patients, and the protocol for this study was approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital.
Cells and cell culture
L02, QSG7701, PLC/PRF/5, SMMC7721, HepG2, SK-Hep-1, WRL68, Hep3B, Huh7, HCCCLM3, and HCCC97L cell lines were obtained from American Type Culture Collection (Manassas, VA) or Cell bank of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in DMEM (GiBco BRL, Life Technologies) with 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere.
Immunohistochemistry
Histological diagnoses of tumourous and non-tumourous formalin-fixed and paraffin-embedded tissues were confirmed on haematoxylin and eosin-stained sections. The tissue sections (3-μm thick) were dewaxed, rehydrated and then immersed in methanol containing 0.3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Subsequently, the sections were heated in a pressure cooker filled with 10 mM ethylenediaminetetracetic acid (EDTA) buffer (pH 8.0) for 2 min. After cooling, the sections were incubated in 1% blocking serum for 30 min to reduce nonspecific binding. Primary anti-ZBTB20 polyclonal antibodies[39] were diluted 1:350 and incubated with the sections at 4°C overnight. Following incubation with biotinylated secondary antisera, the streptavidin-biotin complex/horseradish peroxidase was applied. Finally, the visualization signal was developed with diaminobenzidine (DAB) and the slides were counterstained in hematoxylin.
Stained sections were evaluated in a blinded manner without prior knowledge of the clinical information using the German immunoreactive score, Immuno-Reactive-Score (IRS). Briefly, the IRS assigns sub-scores for immunoreactive distribution (0-4) and intensity (0-3), then multiplies them to yield the IRS score. The percent positivity was scored as "0" (<5%), "1" (5-25%), "2" (25-50%), "3" (50-75%,), "4" (>75%). The staining intensity was score as "0" (no staining), "1" (weakly stained), "2" (moderately stained), and "3" (strongly stained). The final ZBTB20 expression score was calculated with the value of percent positivity score plus staining intensity score, which ranged from 0 to 12 (Figure 1). We estimated IRS by averaging the values in eight fields at ×400 magnification for each specimen. Intratumoral/peritumoral ZBTB20 expression was defined as follows: low expression (score 0-6/0-3) and high expression (score > 6/4-6). Immunohistochemical analysis and scoring were performed by 2 independent investigators.
Typical scored immunohistochemical staining of liver tissue specimens. Formalin-fixed and paraffin-embedded tissues were incubated with primary anti-ZBTB20 polyclonal antibodies (diluted 1:350) at 4°C overnight. The visualization signal was developed with diaminobenzidine (DAB) and the slides were counterstained in hematoxylin. Total score was calculated from sub-scores of staining distribution (0-4) and intensity (0-3).
RNA extraction, cDNA synthesis and real-time RT-PCR
Total RNA was extracted from frozen tissue specimens (50-100 mg) using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. The RNA was reverse transcribed using RT-PCR kits (Applied Biosystems, Foster City, CA, USA) with an oligo d (T) primer under standard conditions. Real-time ZBTB20 PCR amplification was performed in 1× Universal Master Mix (Applied Biosystems) with gene-specific primers and probe on the ABI Prism 7300 Sequence Detection System, according to the manufacturer's instructions. The 18s rRNA levels were quantified as internal control to normalize the expression of each gene. Each reaction was repeated independently at least three times. The following primers were used: ZBTB20, sense 5'- ATGCTAGAACGGAAGAAACCCA -3', antisense 5'-TGTGAGCGTGAGAGTTTGTCA -3';18s, sense 5'-GGGAGGTAGTGACGAAAAA T-3' and antisense 5'-ACCAACAAAATAGAACCGCG-3'. Relative expression levels of genes were calculated and expressed as 2ΔCt.
Western blotting analysis
Western blotting was performed as previously described [41]. In brief, the tissues or cells were lysed in RIPA buffer, sonicated, and protein concentrations calculated by Bicinchoninic acid (BCA) (Sigma). About 60 μg protein samples were run on a 12% SDS-PAGE gel and transferred to a polyvinylidine difluoride membrane. Membranes were blocked for 1 h in Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% skim milk. The membrane was probed with ZBTB20 antibody (dilution, 1:2000) overnight followed by washing triplely with TBST for 5 min and incubated with an IRDye 800CW-conjugated secondary antibody. After being washed three times, the membrane was detected with LI-COR imaging system (LI-COR Biosciences). Mouse anti-human β-actin antibody (dilution, 1:1000; Santa Cruz) was applied as an internal control. The signal intensity of each band on scanned autoradiograms was analysed using LI-COR imaging system. We normalised each sample to the intensity of the β-actin band, and then estimated the value as an expression index.
Follow-Up
Follow-up data were obtained for all the 102 patients with immunohistochemical analysis. The follow-up period was defined as the interval from the date of operation to the date of death or the last follow-up. Deaths from other causes were treated as censored cases. Recurrence and metastasis were diagnosed by clinical examination, AFP measurement, liver ultrasonography, and computed tomography (CT) scan. All patients were observed until December 2009, ranged from 29 to 103 months (median, 73 months). Overall survival (OS) was defined as the interval between the dates of surgery and death. Disease-free survival (DFS) was defined as the interval between the dates of surgery and recurrence; if recurrence was not diagnosed, patients were censored on the date of death or the last follow-up.
To determine factors influencing survival after operation, 13 conventional variables together with ZBTB20 expression were tested in 102 patients: age (≤50 years vs >50 years), gender, tumor size (≤50 mm vs >50 mm), serum AFP level (≤20 ng/mL vs >20 ng/mL), HBsAg (positive vs negative), Edmondson-Steiner grade (I-II vs III-IV), liver cirrhosis (yes vs no), tumor encapsulation (complete vs uncomplete vs no), vein invasion (yes vs no), tumour multiplicity (solitary vs multiple), TNM stage (I-II vs III), recurrence (yes vs no), metastasis (yes vs no), and ZBTB20 intratumoral/peritumoral protein expression level (high vs low).
Statistical analysis
A paired-samples t-test was used to compare the mRNA and protein expression of ZBTB20 between HCC tumorous and the pair-matched non-tumor tissues samples. The Pearsontest or Fisher's exact test was used to analyze the relationship between ZBTB20 expression and the clinicopathologic features. The correlation between the expression of ZBTB20 and AFP was explored using Spearman's correlation analysis. Survival curves were calculated using the Kaplan-Meier method and compared by the log-rank test. The Cox proportional-hazard regression model was used for univariate and multivariate analyses to explore the effect of the clinicopathological variables and ZBTB20 expression on survival. SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses and a p value < 0.05 was considered significant.
Results
Increased Expression Levels of ZBTB20 Messenger RNA and Protein in Hepatocellular Carcinoma
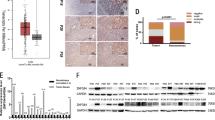
Our previous work showed that ZBTB20 was developmentally up-regulated in liver and was a key repressor governing AFP gene transcription in postpartum liver, and may play an essential role in liver intrinsic functions by regulating genes such as P450 family members, glucose metabolism and the regulation of the somatotropic hormonal axis. To investigate the expression of ZBTB20 and its possible role in HCC, we first detected the protein expression in three human normal liver cell line (L02, QSG7701, WRL68) and 8 HCC cell lines (PLC/PRF/5, SMMC7721, HepG2, SK-Hep-1, Hep3B, Huh7, HCCCLM3, and HCCC97L). ZBTB20 was high expressed in most of the cells (Figure 2A, the whole image was shown in additional file 1). We further performed real-time RT-PCR for the transcriptional level of ZBTB20 from 50 frozen paired samples derived from patients who had undergone hepatectomy for histologically proven HCC. The mRNA level of ZBTB20 was significantly higher in 38 (76%) HCC tissues (tumour; T) compared with the pair-matched non-tumor tissues (NT). We calculated the copy number ratio of ZBTB20 mRNA/18s mRNA. This ratio was significantly higher in tumor samples than in NT samples (4.47 ± 2.71 versus 2.32 ± 0.64, P = 0.006) (Figure 2B).
ZBTB20 expression in HCC tissues and HCC cell lines. A. Western blot analysis of ZBTB20 in HCC cell and normal liver cell lines. B. Evaluation of ZBTB20 mRNA in HCC. C. Western blot analysis of ZBTB20 in HCC and normal liver tissues. T Tumor tissue; NT non-tumor tissue; NL normal liver. D. Expression index of ZBTB20 protein. ZBTB20 protein expression level in HCC was significantly higher than those in NT (P < 0.001). The data are representative of three independent experiments.**: T compared with NT or NL, p <0.05.
Next, the protein level of ZBTB20 in T and NT specimens of 50 HCC cases was analyzed by western blot analysis. ZBTB20 signal was found to be positive in all T samples, and in 78% of NT samples. The intensity of each protein band was normalised against the expression of β-actin in each sample, and this ratio was treated as an expression index. Consistent with mRNA expression, the protein level of ZBTB20 in HCC tumor tissues was also significantly higher than that in NT samples (1.81-fold on average, P < 0.001) (Figure 2C).
Immunohistochemistry Analysis of ZBTB20 Expression and its Relationship with the Clinicopathological Parameters
To investigate the effect and the prognostic value of ZBTB20, immunohistochemistry was used to assess the expression of ZBTB20 in the HCC tissues sections. It was showed that ZBTB20 staining was mainly in the nucleus of tumor cells, partly in the cytoplasm. ZBTB20 expression in normal hepatocytes from para-tumoral tissue of hemangiomas of liver and cirrhotic liver were negative or weakly positive (Figure 3A,B), while mainly moderate or strong positive in HCC tumors (Figure 3C). Overall, 42 of the 102 (41.2%) cases showed high expression (IRS over 6) ZBTB20 in the tumorous tissues, while 60 (58.8%) of the cases showed low expression (IRS 0-6). Generally, ZBTB20 density was significantly higher in intratumoral tissues than in peritumoral tissues (6.37 ± 3.17 versus 2.73 ± 1.68, P < 0.001) (Table 2). Additionally, a sharp contrast was often observed between infiltrative tumorous areas of positive staining and the adjacent nontumorous area of negative staining (Figure 3D).
Immunohistochemistry detection of ZBTB20 expression in normal liver tissue, cirrhotic liver tissue, HCC (T) and in noncancerous hepatic tissues (N). A. Normal liver tissue. B. Cirrhotic liver tissue. The expression of ZBTB20 was weakly positive in hepatocytes. C. HCC cells stained strongly positive with a large granular pattern. D The boundary area between the tumor and non-tumor lesion. Hepatocytes adjacent to HCC lesion were stained with a small granular pattern. Original magnification, ×200. T, tumor; N, Paired-nontumor tissue.
We analysed the relationship between ZBTB20 protein expression and clinical features of HCC by establishing two groups with high and low ZBTB20 expression respectively based on the results of immunohistochemistry analysis. As shown in Table 3, intratumoral ZBTB20 density were positively correlated with vein invasion, recurrence and metastasis. Meanwhile, patients with high peritumoral ZBTB20 density were prone to recurrence. There was no significant association between ZBTB20 expression and the other clinical features, such as age, gender, tumor size, serum AFP level, HBsAg, Edmondson-Steiner grade, liver cirrhosis, tumor encapsulation, tumour multiplicity or TNM stage.
Our past work showed that ZBTB20 negatively regulated AFP [38]. However, in this work, no correlation between serum AFP level and tissue ZBTB20 protein expression was found. Considering the possible discrepancy of serum and liver tissue AFP level, we examined possible relationship between tissue AFP staining level and ZBTB20 expression level in HCCs, adopting Spearman's correlation analysis. In 102 samples, 61.8% cases (63/102) showed high IRS of ZBTB20 with low IRS of AFP; 29.4% cases (30/102) showed low IRS of ZBTB20 with high IRS of AFP; and 8.8% cases (9/102) showed almost the same level of IRS. As shown in Figure 4, the ZBTB20 level was negatively correlated with the staining degree of AFP in HCCs (r = -0.33, P = 0.001), while the serum AFP level had no significant correlation with the tissue AFP IRS (P= 0.538).
Expression of ZBTB20 in HCC and correlation with AFP expression. A. The consecutive slices of two samples immunostained by anti-ZBTB20 or anti-AFP antibody. a&b. Positive staining of AFP in HCC, while negative staining of ZBTB20; c&d. Positive staining of ZBTB20 in HCC, while negative staining of AFP. Magnification: ×100 & ×200. B. Scatter plot of the correlation of IRS of ZBTB20 with AFP. By Spearman's correlation analysis, IRS of ZBTB20 was negatively correlated to AFP (r = - 0.33, P = 0.001).
Correlation between ZBTB20 Expression and Prognosis
At the time of the last follow-up, 74 patients had tumor recurrence, and 59 patients had died. The 1-, 3-, and 5-year OS rates were 77.5%, 53.5%, and 45.9%, respectively; and the 1-, 3-, and 5-year DFS rates were 52.8%, 33.4%, and 26.5%, respectively. The median OS and DFS time were 18 months (95%CI, 1.06 months to 34.94 months) and 6 months (95%CI, 3.98 months to 8.01 months), respectively, for patients with high intratumoral ZBTB20 density (IRS over 6, as described in Materials and methods) and were statistically shorter than the median OS and DFS time for patients with low peritumoral ZBTB20 density [67 months (95%CI, 52.53 months to 81.47 months) and 31 months (95%CI, 13.89 months to 48.12 months), respectively (P = 0.001 and P<0.001, respectively; Figure 5A and 5B)]. Besides, patients with high peritumoral ZBTB20 had poor DFS (9 months vs 22 months, P = 0.002; Figure 5D), whereas peritumoral ZBTB20 was not associated with OS (P = 0.225; Figure 5C)
Cumulative overall and disease-free survival curves of patients with high or low intratumoral or peritumoral features. (A and B) Low intratumoral ZBTB20 was associated with prolonged overall and disease-free survival (P = 0.001 and P<0.001, respectively). (C and D) Low peritumoral ZBTB20 was associated with disease-free survival (P = 0.002) but not with overall survival(P = 0.225).
Univariate Cox regression analyses determined that tumor size, serum AFP, TNM stage, tumor number, tumor encapsulation, vein invasion and intratumoral ZBTB20 expression were significantly associated with OS; while tumor size, HBsAg positive, TNM stage, tumor number, vein invasion and intratumoral/peritumoral ZBTB20 expression were associated with DFS. Furthermore, multivariate Cox regression analyses revealed that high intratumoral ZBTB20 was independent risk factor for OS (relative risk [RR] = 1.952, P = 0.016) and DFS (RR = 2.094, P = 0.003) (Table 4). Besides, tumor size large than 50 mm and vein invasion were also independent predictors for the OS of HCC patients (P = 0.006 and 0.086, respectively; Table 4), while the others failed to show this independence.
Using 12 months as the cutoff value, all of the recurrences were divided into early recurrence, which is mainly from intrahepatic metastasis, and late recurrence, which is usually a result of a multicentric new tumor [42]. Patients with higher intratumoral ZBTB20 density were prone to have an early recurrence compared with patients with low intratumoral ZBTB20 (7.72 ± 2.9 vs 5.01 ± 2.86, P<0.001) (Table 5).
Discussion
In this study, we examined ZBTB20 expression profiles and its correlations with clinicopathologic parameters and prognosis in HCC. Our data revealed that both mRNA and protein levels of ZBTB20 were significantly higher in HCC tissues than in the corresponding non-tumor tissues and normal liver tissues.
We observed that increased expression of ZBTB20 in HCC was positively correlated with tumor vein invasion, recurrence and metastasis. Moreover, patients with high intratumoral ZBTB20 expression had significantly worse OS and DFS when compared with patients with low expression of ZBTB20. Multivariate analysis demonstrated that among the factors analyzed, intratumoral ZBTB20 expression was an independent prognostic factor for OS and DFS in patients with HCC. Meanwhile, tumor size, serum AFP, TNM stage, tumor number and vein invasion were all independent prognostic factors for OS; while tumor size, HBsAg positive, TNM stage, tumor number, vein invasion and peritumoral ZBTB20 expression were independent prognostic factors for DFS. These results clearly demonstrated that high ZBTB20 expression is associated with poor progression and unfavorable clinical outcome of HCC. This showed that high ZBTB20 expression in HCC is an indicator of poor prognosis.
Our earlier work demonstrated that ZBTB20 was a key repressor governing AFP gene transcription in postpartum liver. In this report, our data didn't show any relationship between ZBTB20 expression and serum AFP value. However, ZBTB20 and AFP expression, both of tumor tissue origin, were really inversely related (Figure 4). This was in keeping with the inconsistant relation between serum and tissue AFP level in HCC cases (unpublished data).
The close relationship between ZBTB20 expression and clinicopathological features predicted that ZBTB20 might boost carcinogenesis and tumor progression. Currently, several BTB/POZ-ZF proteins have been implicated in various cancers. For example, the BCL6 and PLZF proteins are causally involved in several types of B-cell lymphomas and acute promyelocytic leukaemia [6–11]. NAC-1 is significantly overexpressed in ovarian serous carcinomas and intense NAC-1 immunoreactivity in primary tumors predicts early recurrence [26] whereas transgenic mice that overexpress Pokemon develop aggressive tumours [15]. Among these proteins, their BTB/POZ domains play a critical role in carcinogenesis and tumor progression. Some researches suggested that targeting the BTB/POZ domain of BCL-6 caused apoptosis and cell cycle arrest, providing a novel approach for cancer therapy [7]. More recently, Cerchietti LC et al found that shock protein 90 (Hsp90) inhibitors selectively kill a subset of Diffuse Large B-cell Lymphomas (DLBCL) that are biologically dependent on the Bcl6 transcriptional repressor, through interrupting direct interaction between BCL-6 and Hsp90 followed by accelerated BCL-6 degradation [43].
Conclusion
We demonstrated here, that ZBTB20 is overexpressed in a large proportion of patients with HCC and high ZBTB20 expression correlated with the disease progression and poor clinical outcome in HCC. Furthermore, ZBTB20 proved to be a risk factor for tumor recurrence and independent molecular marker of prognosis in HCC and may become a novel molecular target in the strategies for the prediction of tumor recurrence and prognosis or treatment of HCC.
Abbreviations
- HCC:
-
Hepatocellular Carcinoma
- BTB/POZ domain ZBTB20:
-
Broad complex, tramtrack, and bric a brac, poxvirus and zinc finger
- AFP:
-
α feto-protein
- IHC:
-
immunohistochemistry
- HBV:
-
Hepatitis B virus
- DAB:
-
Diaminobenzidine
- BSA:
-
Bovine serum albumin
- EDTA:
-
Ethylenediaminetetracetic acid
- IRS:
-
Immuno-Reactive-Score
- BCA:
-
Bicinchoninic acid
- PBS:
-
Phosphate buffered solution
- TBS(T):
-
Triethanolamine-buffered saline(Tween-20)
- Tris:
-
Tris(hydroxymethyl)aminomethane
- SDS:
-
Sodium dodecyl sulphate
- OS:
-
Overall survival
- DFS:
-
Disease-free survival.
References
Max Parkin D, Freddie Bray, Ferlay J, Paola Pisani: Global Cancer Statistics, 2002. CA Cancer J Clin. 2005, 55: 74-108. 10.3322/canjclin.55.2.74.
El-Serag Hashem, Klenhard Rudolph: Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology. 2007, 132: 2557-2576. 10.1053/j.gastro.2007.04.061.
Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D: The BTB/POZ domain: a new protein-protein interaction motif common to DNA-and actin-binding proteins. Cell Growth Differ. 1995, 6 (9): 1193-1198.
Kelly Kevin, Daniel Juliet: POZ for effect - POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 2006, 16 (11): 578-587. 10.1016/j.tcb.2006.09.003.
Stogios PJ, Downs GS, Jauhal JJ, Nandra SK, Privé GG: Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6: R82-10.1186/gb-2005-6-10-r82.
Polo JM, Dell'Oso T, Ranuncolo SM, Cerchietti L, Beck D, Da silva GF, Prive Gibert G, Licht JD, Melnick A: Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004, 10: 1329-1335. 10.1038/nm1134.
Phan RT, Dalla-Favera R: The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004, 432: 635-639. 10.1038/nature03147.
Margalit O, Amram H, Amariglio N, Simon AJ, Shaklai S, Granot G, Minsky N, Shimoni A, Harmelin A, Givol D, Shohat M, Oren M, Rechavi G: BCL6 is regulated by p53 through a response element frequently disrupted in B-cell non-Hodgkin lymphoma. Blood. 2006, 107: 1599-1607. 10.1182/blood-2005-04-1629.
Ranuncolo SM, Polo JM, Dierov J, singer M, Kuo T, Greally J, Green R, Carroll M, Melnick A: Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007, 8: 705-714. 10.1038/ni1478.
Yeyati PL, Shaknovich R, Boterashvili S, Li J, Ball HJ, Waxman S, Burchenal KN, Dmitrovsky ED, Zelent A, Licht JD: Leukemia translocation protein PLZF inhibits cell growth and expression of cyclin A. Oncogene. 1999, 18: 925-934. 10.1038/sj.onc.1202375.
Costoya JA, Pandolfi PP: The role of promyelocytic leukemia zinc finger and promyelocytic leukemia in leukemogenesis and development. Curr Opin Hematol. 2001, 8: 212-217. 10.1097/00062752-200107000-00006.
Felicetti F, Errico MC, Bottero L, Segnalini P, Stoppacciaro A, Biffoni M, Felli N, Mattia G, Petrini M, Colombo MP, Peschle C, Care A: The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008, 68: 2745-2754. 10.1158/0008-5472.CAN-07-2538.
Shiraishi K, Yamasaki K, Nanba D, Inoue H, Hanakawa Y, Shirakata Y, Hashimoto K, Higashiyama S: Pre-B-cell leukemia transcription factor 1 is a major target of promyelocytic leukemia zinc-finger-mediated melanoma cell growth suppression. Oncogene. 2007, 26: 339-348. 10.1038/sj.onc.1209800.
Parrado A, Robledo M, Moya-Quiles MR, Marin LA, Chomienne C, Padua RA, Alvarez-lopez MR: The promyelocytic leukemia zinc finger protein down-regulates apoptosis and expression of the proapoptotic BID protein in lymphocytes. Proc Natl Acad Sci USA. 2004, 101: 1898-1903. 10.1073/pnas.0308358100.
Maeda T, Hobbs RM, Merghoub T, Merghoub T, Guernah I, Zelent A, Cordon-cardo C, Teruya-Feldstein , Pandolfi PP: Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005, 433: 278-285. 10.1038/nature03203.
Maeda T, Hobbs RM, Pandolfi PP: The transcription factor Pokemon: a new key player in cancer pathogenesis. Cancer Res. 2005, 65: 8575-8578. 10.1158/0008-5472.CAN-05-1055.
Aggarwal A, Hunter WJ, Aggarwal H, Silva ED, Davey MS, Murphy RF, Agrawal DK: Expression of leukemia/lymphoma-related factor (LRF/POKEMON) in human breast carcinoma and other cancers. Exp Mol Pathol. 2010, 89 (2): 140-8. 10.1016/j.yexmp.2010.05.002. Epub 2010 May 21
Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, R Brink MRM, Zelent A, Shigematsu H, Akashi K, Teruya-Feldstein J, Cattoretti G, pandolfi PP: Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007, 316: 860-866. 10.1126/science.1140881.
Mondal AM, Chinnadurai S, Datta K, Chauhan SS, Sinha S, Chattopadhyay P: Identification and functional characterization of a novel unspliced transcript variant of HIC-1 in human cancer cells exposed to adverse growth conditions. Cancer Res. 2006, 66: 10466-10477. 10.1158/0008-5472.CAN-06-0352.
Waha A, Waha A, Koch A, Meyer-Puttlitz B, Weqqen S, Sorensen N, Tonn JC, Albrecht S, Goodyer CG, Wiestler OD, Pietsch T: Epigenetic silencing of the HIC-1 gene in human medulloblastomas. J Neuropathol Exp Neurol. 2003, 62: 1192-1201.
Nishida N, Nagasaka T, Nishimura T, Ikai I, Boland CR, Goel A: Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology. 2008, 47: 908-918.
Hayashi M, Tokuchi Y, Hashimoto T, Hayashi S, Nishida K, Ishikawa Y, Nakagawa K, Tsuchiya S, Tsuchiya E: Reduced HIC-1 gene expression in non-small cell lung cancer and its clinical significance. Anticancer Res. 2001, 21: 535-540.
Nicoll G, Crichton DN, McDowell HE, Kernohan N, Hupp TR, Thompson AM: Expression of the Hypermethylated in Cancer gene (HIC-1) is associated with good outcome in human breast cancer. Br J Cancer. 2001, 85: 1878-1882. 10.1054/bjoc.2001.2163.
Mackler SA, Korutla L, Cha XY, Koebbe MJ, Fournier KM, Bowers MS, Kalivas PW: NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J Neurosci. 2000, 20: 6210-6217.
Korutla L, Neustadter JH, Fournier KM, Mackler SA: NAC1, a POZ/BTB protein present in the adult mammalian brain, triggers apoptosis after adenovirus-mediated overexpression in PC-12 cells. Neurosci Res. 2003, 46: 33-39. 10.1016/S0168-0102(03)00024-5.
Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman RJM, Wang TL, Shih leM: A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci USA. 2006, 103: 18739-18744. 10.1073/pnas.0604083103.
Yeasmin S, Nakayama K, Ishibashi M, Claes G: Trope, Tian-li Wang, leming Shih: Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin Cancer Res. 2008, 14: 1686-1691. 10.1158/1078-0432.CCR-07-4085.
Davidson B, Berner A, Trope' CG, Wang TL, Shih IeM: Expression and clinical role of the bric-a-brac tramtrack broad complex/poxvirus and zinc protein NAC-1 in ovarian carcinoma effusions. Hum Pathol. 2007, 38: 1030-1036. 10.1016/j.humpath.2006.12.009.
Nakayama K, Nakayama N, Wang TL, Shih IeM: NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007, 67: 8058-64. 10.1158/0008-5472.CAN-07-1357.
Prokhortchouk A, Sansom O, Selfridge J, Caballero IM, Salozhin S, Aithozhina D, Cerchietti L, Meng FG, Augenlicht LH, Mariadason JM, Hendrich B, Melnick A, Prokhortchouk E, Clarke A, Bird A: Kaiso-deficient mice show resistance to intestinal cancer. Mol Cell Biol. 2006, 26: 199-208. 10.1128/MCB.26.1.199-208.2006.
Van Roy FM, McCrea PD: A role for Kaiso-p120ctn complexes in cancer?. Nat Rev Cancer. 2005, 5: 956-964. 10.1038/nrc1752.
Daniel JM, Reynolds AB: The catenin p120 (ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999, 19: 3614-3623.
Soubry A, van Hengel J, Parthoens E, Colpaert C, Van Marck E, Waltrgny D, Reynolds AB, van Roy F: Expression and nuclear location of the transcriptional repressor Kaiso is regulated by the tumor microenvironment. Cancer Res. 2005, 65: 2224-2233. 10.1158/0008-5472.CAN-04-2020.
Zhang W, Mi J, Li N, Sui L, Wan T, Zhang J, Chen T, Cao X: Identification and Characterization of ZBTB20, a Novel Human BTB/POZ Zinc Finger Protein Sharing Homology to BCL-6. Biochem Biophys Res Commun. 2001, 282: 1067-1073. 10.1006/bbrc.2001.4689.
Mitchelmore C, Kjaerulff KM, Pedersen HC, Nielsen JV, Rasmussen TE, Fisker MF, Finsen B, Pedersen KM, Jensen NA: Characterization of Two Novel Nuclear BTB/POZ Domain Zinc Finger Isoforms. J Biol Chem. 2002, 277: 7598-7609. 10.1074/jbc.M110023200.
Harboe TL, Tümer Z, Hansen C, Jensen NA, Tommerup N: Assignment of the human zinc finger gene, ZNF288, to chromosome 3 band q13.2 by radiation hybrid mapping and fluorescence in situ hybridisation. Cytogenet Cell Genet. 2000, 89: 156-157. 10.1159/000015600.
Nielsen JV, Nielsen FH, Ismail R, Noraberg J, Jensen NA: Hippocampus-like corticoneurogenesis induced by two isoforms of the BTB-zinc finger gene Zbtb20 in mice. Development. 2007, 134: 1133-1140. 10.1242/dev.000265.
Xie Z, Zhang H, Tsai W, Zhang Y, Du Y, Zhong J, Szpirer C, Zhu M, Cao X, Barton MC, Grusby MJ, Zhang WJ: Zinc finger protein ZBTB20 is a key repressor of alpha-fetoprotein gene transcription in liver. Proc Natl Acad Sci USA. 2008, 105: 10859-10864. 10.1073/pnas.0800647105.
Sutherland AP, Zhang H, Zhang Y, Michaud M, Xie Z, Patti ME, Grusby MJ, Zhang WJ: Zinc finger protein Zbtb20 is essential for postnatal survival and glucose homeostasis. Mol Cell Biol. 2009, 29: 2804-2815. 10.1128/MCB.01667-08.
Sobin LH, Gospodarowicz MK, Wittekind CH, Eds: TNM Classification of Malignant Tumors. 2002, New York, Wiley-Liss, Inc., 81-83. 6
Dong LW, Kong XN, Yan HX, Yu LX, Chen L, Yang W, Liu Q, Huang DD, Wu MC, Wang HY: Signal regulatory protein alpha negatively regulates both TLR3 and cytoplasmic pathways in type I interferon induction. Mol Immunol. 2008, 45: 3025-3035. 10.1016/j.molimm.2008.03.012.
Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J: Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000, 89: 500-507. 10.1002/1097-0142(20000801)89:3<500::AID-CNCR4>3.0.CO;2-O.
Cerchietti LC, Lopes EC, Yang SN, Hatzi K, Bunting KL, Tsikitas LA, Mallik A, Robles AI, Walling J, Varticovski L, Shaknovich R, Bhalla KN, Chiosis G, Melnick A: A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med. 2009, 15: 1369-1376. 10.1038/nm.2059.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/11/271/prepub
Acknowledgements and funds
The study has been supported by the State Key Science & Technology Project for Infectious Diseases (2008ZX10002), National Key Project of Scientific and Technical Supporting Programs (2008BAI63B03, 2006AA02A249) from State Ministry of Science & Technology of China, and the foundation (30772478, 30921006, 90713032, 06MA154) from National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
QW conducted the study and drafted the manuscript. YT conceived and designed the study. YR conducted the study and collected the clinical data. LD, ZX, LT, DC and WZ analyzed and interpreted the data. HH and HW provided the administrative support. All the authors have read and approved the final version of the manuscript.
Qing Wang, Ye-xiong Tan, Yi-bin Ren contributed equally to this work.
Electronic supplementary material
12885_2011_2763_MOESM1_ESM.JPEG
Additional file 1: Whole image of western blot showing ZBTB20 expression. ZBTB20 expression was detected in 4 pairs of HCC specimens, 3 HCC cell lines (HepG2, PLC and Huh-7) and 293T cell line transiently transfected with ZBTB20-expressing plasmid as well as its negative control. Molecular Marker. N: Non-tumor T: Tumor. L:293T cell line transiently transfected with ZBTB20-expressing plasmid; R: 293T cell line transiently transfected with empty plasmid. Arrowhead indicates the ZBTB20 ladder. (JPEG 91 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wang, Q., Tan, Yx., Ren, Yb. et al. Zinc finger protein ZBTB20 expression is increased in hepatocellular carcinoma and associated with poor prognosis. BMC Cancer 11, 271 (2011). https://doi.org/10.1186/1471-2407-11-271
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-11-271