Abstract
Background
The formation of metastases includes the separation of tumor cells from the primary tumor, cell migration into subendothelial tissue and cell proliferation in secondary organ. In this process, cell adhesion of tumor cells to the endothelium is an essential requirement for formation of metastases. Protein kinase C (PKC) regulates adhesion and proliferation. To identify a relation between PKC isoforms and tumor progression in renal cell carcinoma (RCC), the influence of PKC isoforms on cell adhesion and proliferation, and possible influences of integrins were analyzed in RCC cells.
Methods
The experiments were performed in the RCC cell lines CCF-RC1 and CCF-RC2 after pre-incubation (16 h) with the PKC inhibitors GF109203X (inhibits PKCα, βI, βII, γ, δ and ε), GÖ6976 (inhibits PKCα, βI and μ), RO31-8220 (inhibits PKCα, βI, βII, γ and ε) and rottlerin (inhibits PKCδ). Cell adhesion was assessed through adherence of RCC cells to an endothelial monolayer. Cell proliferation was analyzed by a BrdU incorporation assay. The expression of β1 integrins was analyzed by flow cytometry.
Results
In CCF-RC1 cells, cell adhesion was significantly reduced by GÖ6976 to 55% and by RO31-8220 to 45% of control. In CCF-RC2 cells, only GÖ6976 induced a significant reduction of cell adhesion to 50% of control levels. Proliferation of both cell lines was reduced by rottlerin to 39% and 45% of control, respectively. The β1 integrin expression on the cell surface of CCF-RC1 and CCR-RC2 cells was decreased by RO31-8220 to 8% and 7% of control, respectively. β2 and β3 integrins were undetectable in both cell lines.
Conclusions
The combination of the PKC inhibitors leads to the assumption that PKCμ influences cell adhesion in CCF-RC1 and CCF-RC2 cells, whereas in CCF-RC1 cells PKCε also seems to be involved in this process. The expression of β1 integrins appears to be regulated in particular by PKCε. Cell proliferation was inhibited by rottlerin, so that PKCδ might be involved in cell proliferation in these cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Formation of metastases includes the separation of single cells from the primary tumor, migration into the extracellular matrix, blood vessel invasion, adhesion to endothelium, migration through the endothelium and growth in a secondary organ [1]. During extravasation into the secondary organ, tumor cells seem to undergo the same mechanisms as leukocytes in inflammatory processes. After a loose contact to endothelial cells, integrins on the cell surface of leukocytes become activated by a chemokine induced inside-out signaling sought by endothelial cells [2] or by direct cell-cell contact [3]. Activated integrins, in particular β1, β2 and β3 integrins, mediate a firm adhesion to endothelial cells by binding their ligands such as ICAM, VCAM, PECAM or other integrins [4–6] leading to transendothelial migration. In the process of metastases, the adhesion of tumor cells to endothelial cells has also been shown to be mediated by integrins. The tumor cells bind their ligands, located on the cell surface of endothelial cells, leading to a firm adhesion, and subsequently to transendothelial migration. In vitro experiments showed a major importance in the binding of α4β1 integrin to VCAM in several tumor entities in tumor cell adhesion [7, 8]. Furthermore, α6β1, αvβ1 and αvβ3 integrins have been shown to be involved in tumor cell-endothelial cell adhesion [9–11].
In renal cell carcinoma, an important role has also been demonstrated for β1 integrins [12, 13]. The function of integrins can rapidly be changed by altering their binding affinity for ligands through inside-out signaling. Inside-out signaling induces a conformational change from the cytoplasmic domains in the direction of the extracellular binding site, in response to intracellular signaling events. Signaling molecules involved in inside-out signaling of integrins are G proteins, Ca2+, phospholipase, tyrosine kinase, CaM kinase II, and protein kinases C (PKCs) [14–16]. The activation pathway on integrins by PKC includes RACK (receptor for activated C kinase), which binds to the β subunit of integrins [17]. PKC modulation results in an alteration of the integrin avidity and affinity [18]. In addition to the activity of integrins, PKC regulates the integrin expression on the cell surface [19, 20]. These reports demonstrate the interaction between PKC and integrins.
The family of PKC comprises phospholipid dependent serine/threonine protein kinases deriving from different PKC genes, and from alternative splicing of a single transcript [21]. Up to 10 distinct family members have been discovered in mammalian cells, which are classified into Ca2+-dependent conventional cPKC isoforms α, βI, βII and γ, Ca2+-independent novel nPKCs δ, ε, η and θ, and the atypical aPKCs λ/τ and ζ. PKCμ/PKD, a Ca2+ independent PKC with a unique substrate specificity which differs from the PKC isoforms [22], has primary been related to the PKC family, but cannot be attributed as a member of the PKC family. In contrast to the PKC family, which belongs to the AGC group (PKA, PKG, PKC), PKCμ belongs to the CAMK group (Calcium/calmodulin-dependent protein kinase) [23, 24]. The expression patterns of PKC isoforms differ between tissues and the subcellular distribution of the isoforms varies depending on cell type and physiological condition [25–27], so that an overexpression of the same PKC isoform in different cells may result in opposite biological effects, depending on the cell type [28, 29].
To date, little is known about the isoform specific role of PKC in metastasis of renal cancer. To answer this question, we investigated the influence of different-isoform specific PKC inhibitors on the metastatic behavior of renal carcinoma cells in vitro. Consequently, the involvement of PKC in the adhesion of renal tumor cells, as well as cell proliferation and the expression of integrins was analyzed.
Methods
Cells and cell culture
The human renal carcinoma cell lines CCF-RC1 and CCF-RC2 were used for all experiments. CCF-RC1 was isolated from a high grade pleomorphic renal cell carcinoma (nuclear grade 3) [30, 12], CCF-RC2 was cultured from the effluent of the same kidney. Cell culture was maintained in RPMI 1640 with L-glutamine (Gibco, Eggenstein, Germany), supplemented with 10% fetal calf serum and 0.5% penicillin/streptomycin (Gibco, Eggenstein, Germany). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 in air. Cells in the present study were used between passage 20 and 30.
For cell adhesion experiments, human umbilical vein endothelial cells (HUVEC) were isolated as previously described [31, 32]. Briefly, intact segments of 1 to 3-days-old umbilical cords were drained, and the remaining blood was rinsed from the umbilical vein with phosphate buffered saline (PBS) via a blunt cannula attached at one end of the vessel. The open end of the cord was sealed and the cord distended with 1 mg/ml collagenase II and incubated at room temperature for 30 min. The cord was massaged and cut, and the collagenase digest collected. The cells were washed, seeded into fibronectin-coated flasks (1 μg human fibronectin per cm2 flask) and grown in Medium 199 media supplemented with 20% fetal calf serum (FCS), 25 μg/ml heparin and 25 μg/ml endothelial cell growth supplement (ECGS). Cells were subcultured by trypsin-EDTA treatment and used until the fifth passage in the experiments.
Treatment of renal tumor cells with PKC inhibitors
The renal tumor cells CCF-RC1 and CCR-RC2 were incubated with the PKC inhibitors GF109203X (inhibits PKCα, βI, βII, γ, δ and ε), GÖ6976 (inhibits PKCα, βI and μ), RO31-8220 (inhibits PKCα, βI, βII, γ and ε) (concentrations of 1, 2 and 5 μM) and rottlerin (inhibits PKCδ) (concentrations of 1, 5 and 10 μM, all from Calbiochem, La Jolla, USA) in serum free medium for 16 hours at 37°C in a humidified atmosphere containing 5% CO2 in air. The IC50 values of the inhibitors against PKC isoforms are listed in Table 1. Serum free culture medium without inhibitors served as a control. To analyze toxic effects of PCK inhibitors, renal tumor cells were incubated with PKC inhibitors as described above, each of them fourfold.
MTT cell vitality assay
The mitochondrial activity of the cells was assessed by a MTT assay kit (Sigma, Deisenhofen, Germany). In this assay the vitality of living cells was measured via mitochondrial dehydrogenases. The yellow MTT (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide) was reduced to a blue formazan product by mitochondrial activity. Briefly, after incubation of the cells with PKC inhibitors, culture medium was replaced by MTT solution (0.5 mg/ml in phenol red free medium) and incubated for two hours at 37°C. Afterwards, formazan crystals were dissolved by MTT solubilization solution and measured spectrophotometrically at a wavelength of 570 nm (reference wavelength 690 nm).
Cell adhesion assay
To analyze the adhesion of renal tumor cells to an endothelial monolayer, HUVEC (1.5 × 104 cells/well) were seeded into a 96 well plate coated with 1.5 mg/cm2 gelatine. Cells were allowed to grow to a confluent monolayer within three days. PKC inhibitor-treated and control CCF-RC1 cells were labeled by 10 μM BrdU (Bromodeoxyuridine) for 30 min at 37°C. A cell suspension containing 104 cells was added to the endothelial cell layer under presence of the particular PKC inhibitor and incubated at 37°C in a humidified atmosphere containing 5% CO2 in air. After two hours, non-adherent cells were removed by washing with PBS. To normalize the level of BrdU per cell, a defined number of 104 BrdU labeled tumor cells were centrifuged in parallel into a 96 well plate. The relative amount of BrdU per well was quantified as described in the next section. Each experiment was performed eightfold and repeated three times.
Analysis of cell proliferation
To study the effect of PKC inhibitors on proliferation, a colorimetric BrdU incorporation assay (Roche, Mannheim, Germany) was performed. Each 3 × 103 cells were seeded in triplicate into a 96 well plate and treated in triplicate by PKC inhibitors in serum free medium. BrdU solution (10 μM) was added to the cells and incubated for two hours at 37°C in a humidified atmosphere containing 5% CO2 in air. After removing the culture medium, the cells were fixed and the DNA was denatured in one step by adding fixDenat solution. Incorporated BrdU was detected by an anti-BrdU-POD antibody. The immune complex was detected by a subsequent substrate reaction and quantified by measuring the absorbance at 450 nm (reference wavelength 690 nm).
Western blot analyses
For preparation of protein extracts, semiconfluent CCF-RC1 and CCF-RC2 members were rinsed twice with ice-cold phosphate-buffered saline (PBS) and scraped off the dish with a rubber policeman in lysis buffer on ice (20 mM HEPES, pH 7.7, 0.2 M NaCl, 1.5 mM MgCl2, 0.4 mM EDTA, 1% Triton X-100, 0.5 mM DTT, 100 μg/ml leupeptin, 100 μg/ml aprotinin, 10 mM benzamidine, 2 mM phenylmethylsulphonyl fluoride, 20 mM β-glycerophosphate and 0.1 mM sodium-orthovanadate) [33]. Cell lysate was centrifuged at 14,000 rpm at 4°C for 20 min. Protein concentrations of the supernatants were determined using Bicinchoninic acid reagents. Thirty μg protein/lane protein extract of the cells was separated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) in 10% acrylamide gels and transferred by semi-dry blotting onto polyvinylidene fluoride membranes (PVDF, Immobilon P, Millipore, Bedford, MA). The membrane was incubated with blocking solution (Roth, Karlsruhe, Germany) for 1 h before incubation with the primary antibodies against the PKCδ (78 kDa), PKCε (83 kDa), PKCμ/PKD1 (115 kDa), PDK2 (105 kDa) and PKCν/PDK3 (100 kDa) (all 1:300, Santa Cruz, Heidelberg, Germany) overnight at 4°C. After washing three times, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (Dako, Hamburg, Germany) for 1 h at room temperature. The bound antibodies were visualized by enhanced chemiluminescence (ECL, NEN Lifescience, Boston, USA) detection system using Fuji medical X-ray film.
Flow cytometry
The integrin surface expression of untreated as well as PKC inhibitor treated tumor cells was quantified by flow cytometry. Briefly, cells were harvested in a 0.02% EDTA solution and incubated with a monoclonal FITC labeled anti-CD29 (β1 chain of integrins), anti-CD18 (β2 chain of integrins), anti-CD61 (β3 chain of integrins) or an isotypic control immunoglobuline (all Immunotech, Hamburg, Germany), respectively, for 15 min, at 4°C in darkness. Then, integrin expression was quantified on a flow cytometer (BP Calibur, Becton Dickinson, Heidelberg, Germany).
Statistical analysis
For statistical confirmation, three or four independent adhesion and proliferation experiments, each four- to eightfold, were performed respectively. A mean value and a standard error were calculated from the results. For significance analyses, SPSS 17.0 software was applied. Differences in cell vitality, proliferation and adhesion were performed using the Mann-Whitney test. Differences were considered statistically significant at p < 0.05.
Results
Cell vitality after PKC inhibition
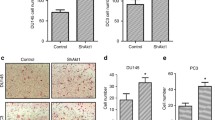
Renal tumor cell vitality was determined by MTT assays. With exception of GÖ6976 in CCF-RC2 cells, treatment of renal cancer cells with the PKC inhibitors GF109203X, GÖ6976, RO31-8220 (1, 2 and 5 μM) and rottlerin (1, 5 and 10 μM) did not influence cell vitality. Treatment of CCF-RC2 cells with GÖ6976 resulted in a non-significant reduced vitality to 68% of control (Figure 1).
Cell vitality after PKC inhibition. CCF-RC1 (A) and CCF-RC2 (B) cells were treated with PKC isoform specific inhibitors GF109203X, GÖ6976, RO31-8220 and rottlerin in different concentrations, cell vitality was determined by MTT assay. Only treatment of CCF-RC2 cells with GÖ6976 resulted in a slight reduction of cell vitality. The columns and bars represent mean value and standard error in % of control (untreated cells).
Cell proliferation after PKC inhibition
To assess the influence of PKC inhibition on cell proliferation, CCF-RC1 and CCF-RC2 cells were incubated with the PKC inhibitors GF109203X, GÖ6976, RO31-8220 and rottlerin in concentrations as described. Proliferation was determined by BrdU incorporation. In CCF-RC1 and CCF-RC2 cells we observed reduced cell proliferation after treatment with RO31-8220 and rottlerin in a concentration dependent manner. RO31-8220 led to 58% (CCF-RC1, p = 0.057) and 68% (CCF-RC2, p = 0.012) of control cell proliferation. After rottlerin treatment, cell proliferation was reduced to 37% p = 0.029) and 45% (p = 0.012) of control measurements in CCF-RC1 and CCF-RC2 cells, respectively. In CCF-RC1 cells, also treatment with GÖ6976 resulted in a slightly reduced cell proliferation to 74% of control (p = 0.200, Figure 2).
Cell proliferation after PKC inhibition. CCF-RC1 (A) and CCF-RC2 (B) cells were treated with the PKC isoform specific inhibitors GF109203X, GÖ6976 and RO31-8220 in concentrations between 1 μM and 5 μM, and with Rottlerin in concentrations between 1 μM and 10 μM. The proliferation value is determined as a percentage of the proliferation of control (untreated cells). The columns and bars represent mean value and standard error in % of control.
Cell adhesion of PKC inhibitor treated renal tumor cells to endothelial cells
For investigation of the adhesion of renal carcinoma cells to endothelial cells, CCF-RC1 and CCF-RC2 cells were treated with PKC inhibitors GF109203X, GÖ6976, RO31-8220 and rottlerin as described and the adhesion of these cells to a monolayer of HUVECs was determined. In CCF-RC1 cells, incubation with GÖ6976 and RO31-8220 resulted in a concentration dependent inhibition of cell adhesion to 55% (p = 0.029) and 45% (p = 0.029) of control cells, respectively. In contrast, CCF-RC2 cells showed a reduced tumor cell - endothelial cell adhesion after treatment also with GÖ6976 (50% of control, p = 0.029), whereas RO31-8220 resulted in no alteration of cell adhesion (Figure 3).
Cell adhesion to endothelial cells after PKC inhibition. Adhesion of CCF-RC1 (A) and CCF-RC2 (B) cells to a monolayer of human umbilical endothelial cells (HUVEC) was measured after treatment with the PKC isoform specific inhibitors GF109203X, GÖ6976 and RO31-8220 in concentrations between 1 μM and 5 μM, and with Rottlerin in concentrations between 1 μM and 10 μM. The adhesion value is determined as a percentage of the adhesion of control (untreated cells). The columns and bars represent mean value and standard error in % of control.
Influence of PKC inhibitors on the expression of β1 integrins
Since tumor cell adhesion to endothelial cells by integrins is mediated in the first line by β1, β2 and β3 integrins [34], the expression of these integrins was determined by flow cytometry analysis. Both cell lines, CCF-RC1 and CCF-RC2 cells, showed a clear expression of β1 integrins, whereas neither β2 nor β3 integrins were expressed in these renal tumor cells (Figure 4). In CCF-RC1 cells, β1 integrin levels were impaired by all PKC inhibitors used in altered extent. GF109203X induced a reduction of β1 integrin expression to 53%, GÖ6976 to 52%, RO31-8220 to 8% and rottlerin to 73% as compared to controls, respectively. In CCF-RC2 cells, only GF109203X and RO31-8220 treatment resulted in a 35% and 7% reduced β1 integrin expression as compared to control expression levels (Figure 5). In our experiment, RO31-8220 was the strongest inhibitior of β1 integrins.
Expression of β1 integrins after PKC inhibition. CCF-RC1 (A) and CCF-RC2 (B) cells were treated with the PKC isoform specific inhibitors GF109203X, GÖ6976 and RO31-8220 in concentrations between 1 μM and 5 μM, and with Rottlerin in concentrations between 1 μM and 10 μM, integrin expression was determined by flow cytometry. The columns represent mean value of 10 000 counted cells in % of control (untreated cells).
Expression of PKCδ, PKC and, PKCμ in CCF-RC2 cells and PKD2 and PKD3 in both cell lines
In former investigations we showed an expression of PKCδ, ε and μ in CCF-RC1 cells [19]. Since these three isoforms seem to be relevant in metastatic processes of renal cancer cells, we analyzed their expression in CCF-RC2 cells. Furthermore, PKC2 and 3 were analyzed in both cell lines. Our Western blot analyses showed a strong expression of PKCδ and, to a lesser extent, PKCε and μ in CCF-RC2 cells (Figure 6). PKC2 and 3 (PKCν) were not detectable in either cell line (data not shown).
Discussion
Renal cell carcinoma is a tumor entity with a high risk for metastases and consequently carries a poor prognosis. Therefore, investigations are based on the analysis of metastatic mechanisms. Adhesion of tumor cells to the endothelium of blood vessels during the process of extravasation is one of the most important prerequisites for the formation of metastasis. We investigated the influence of various PKC isoforms on the regulation of endothelial cell adhesion of the renal carcinoma cell lines CCF-RC1 and CCF-RC2. The cells were incubated with PKC isoform specific inhibitors GF109203X (inhibits PKCα, βI, βII, γ, δ and ε), GÖ6976 (inhibits PKCα, βI and μ), RO31-8220 (inhibits PKCα, βI, βII, γ and ε) and rottlerin (inhibits PKCδ) [35]. Afterwards we quantified cell adhesion to endothelial cells, cell proliferation and the expression of β1, β2 and β3 integrins. In contrast to our former investigations [36], we pre-incubated the cells with the PKC inhibitors for 16 hours. This long-time incubation not only can lead to regulatory response but also allows intracellular changes in gene expression.
In CCF-RC1 cells treatment with GÖ6976 and RO31-8220 resulted in a clearly reduced adhesion to endothelial cells (55% and 45% of control, respectively). Treatment of CCF-RC2 cells with GÖ6976 also led to a reduced cell adhesion (50%), whereas RO31-8220 treatment had no impact on cell adhesion in this cell line. The reducing effect of GÖ6976 on CCF-RC2 cells must be interpreted with caution, since our MTT test, which determines the influence of the inhibitor on cell vitality, also showed a slightly inhibitory effect of GÖ6976 in CCF-RC2 cells (Figure 1B). The distinct inhibitory effect of GÖ6976 in CCF-RC2 cells could partly be a result of the cell toxicity of this agent, causing a general reduction in cell vitality and consequently reduced cell adhesion. However, GÖ6976 reduced tumor cell adhesion to endothelial cells in both cell lines. The only PKC isoform which is inhibited by GÖ6976, but not by GF109203X, nor RO31-8220 or rottlerin, is PKCμ. PKD2 and PKCν (PKD3) are also inhibited by GÖ6076, although no isoform specific IC50 values for these PKD isoforms have been published. Because PKD2 and PKCν/PKD3 are not expressed in the cell lines tested, our results lead to the assumption that PKCμ is involved in the regulation of adhesion of the renal cancer cells CCF-RC1 and CCF-RC2.
PKCμ, also called PKD1 (protein kinase D), and additionally classified as a member of a new protein kinase family [23], has been shown to be activated dependent on PKCε or PKCη [37, 38]. In cancer cells, PKCμ is known to be involved in metastasis. During this process, PKCμ forms a complex with paxillin and cortactin, leading to the formation of invadopodia, initiating cell migration and degradation of the basement membrane [39]. In this context, PKCμ mediated cortactin phosphorylation [40]. On the other hand, the loss of PKCμ expression increases the malignant potential of breast cancer cells [41]. Syed et al. showed that an overexpression of the subtype PKCμ1 in prostate carcinoma cells increased cell aggregation and decreased cell motility [42]. This PKC isoform has been shown to phosphorylate β-catenin, and consequently also influences cell motility [43]. In accordance with an important role in cell motility, PKCμ also mediates integrin recruitment to newly formed focal adhesions by promoting the recycling of αvβ3 integrin [44]. However, our study did not show any expression of β3 integrins in CCF-RC1 and CCF-RC2 renal cancer cells; therefore, this mechanism could not be studied.
In CCF-RC1 cells, treatment with GO6976 resulted in a reduced expression of β1 integrins, leading to the assumption that the effect of PKCμ on cell adhesion occurs via β1 integrins. In contrast, no reduced β1 integrin expression was detectable in CCF-RC2 cells. Assuming that this effect is not caused by a general reduced cell vitality induced by GO6976, and as detected by the MTT test performed in this study, a further cell adhesion mechanism seems to take place in this cell line. The adhesion of tumor cells to endothelial cells is also regulated by other cell adhesion molecules. The most significant integrin-independent mechanism between the interaction of tumor cells and endothelial cells is the binding of sialyl-Lewis × on the surface of tumor cells to E-selectin on endothelial cells. The expression of sialyl-Lewis × in tumor cells is regulated by members of the α1,3 fucosyltransferase (α1,3FuT) family, and in particular by FuT6 [45]. PKCμ has the ability to regulate the expression of FuT by inducing NFκB [46]. In this way, an inhibition of PKCμ should reduce the expression of sLeX on the RCC cells. Since the expression of sLeX on CCF-RC2 cells was less extensive compared to CCF-RC1 cells [47], these cells may be more sensitive to inhibition of sLeX. These mechanisms must be clarified in further investigations.
In CCF-RC1 cells, in addition to GÖ6976, RO31-8220 also reduced adhesion to endothelial cells. PKCε is reduced by RO31-8220 (IC50: 24 μM), but to a much lesser extend by GF109203X (IC50: 132 μM), and not by GÖ6976 or rottlerin. None of the PKC isoforms are inhibited by GÖ6976 and RO31-8220, and not inhibited by GF109203X or rottlerin at the same time (Table 1). Therefore, in CCF-RC1 cells, in addition to PKCμ, PKCε also seems to regulate the adhesion to endothelial cells. Iaska and coworkers described a functional relationship between PKCε activity and the membrane trafficking of β1 integrins in fibroblasts [48]. After activation, PKCε translocates to the cell membrane, where it colocalize with β1 integrins at membrane ruffles. Furthermore, inhibition of PKCε leads to accumulation of β1 integrins in vesicles, suggesting a PKCε dependent step in the trafficking of integrins [48]. Integrin trafficking is important for recycling after internalisation. Impaired PKCε dependent recycling reduces integrin expression on the cell membrane. For this reason, we analyzed the expression of integrins on the cell surface of CCF-RC1 and CCF-RC2 cells. And indeed, treatment with RO31-8220, in contrast to the other PKC inhibitors used, showed a strong inhibition of β1 integrin expression to 8% and 7% of control levels, respectively. This confirms earlier results showing a reduced β1 integrin expression after short time treatment of renal cancer cells with RO31-8220 [19]. Therefore, the deficient recycling of β1 integrins after treatment of tumor cells with RO31-8220 may offer an explanation for the observation made in the present study, i.e. that PKCε inhibition in both cell lines tested results in a reduced expression of β1 integrins on the cell membrane. Other authors also showed an involvement of PKCε in cell adhesion and proliferation. For instance, PKCε appears to be responsible for the regulation of HeLa cell adhesion to the extracellular matrix [20]. It is also implicated in β1 integrin-mediated adhesion of human breast carcinoma cells to collagen [49]. In mammary carcinoma cells PKCε as well as PKCμ have been shown to regulate cell adhesion to collagen IV in dependence of β1 integrins [44]. Furthermore, Engers and coworkers suggested a role of PKCε in invasion of renal carcinoma cells using a chick heart invasion assay [50]. However, in contrast to others, this study demonstrated a PKCε dependent adhesion to endothelial cells.
The two cell lines investigated differ in origin, although both were obtained from the same patient. CCF-RC1 was established from the primary tumor and CCF-RC2 from cells of the renal vein effluent of the perfused tumorous kidney [30]. Both have been characterized as renal carcinoma cells with similar characteristics, although CCF-RC2 cells have already performed the first step of metastasis, the detachment from the combined cell structure and migration from the primary tumor. For this reason, these cells are possibly more resistant against PKC inhibitors, which may explain their different behavior.
Although PKCμ and PKCε are known to influence cell proliferation [51–53], our investigations demonstrate a distinct alteration in cell proliferation solely after treatment of renal cancer cells with rottlerin, a PKCδ inhibitor. PKCδ is known to be involved in regulation of the cell cycle and apoptosis, and has also been shown to play a role in renal cell carcinoma [54]. PKCδ induces cell cycle arrest in many cell types by inhibiting the expression of cyclin D1 and cyclin E and up-regulating p27 [55–57]. Additionally, PKCδ activates DNA-PK (DNA dependent protein kinase) [58], antagonizes the Jak-STAT pathway and inhibits phospholipase D, an antagonist of Raf [59]. On the other hand, an inhibition of PKCδ induces apoptosis in B chronic lymphocytic leukemia cells, whereas in healthy B cells a more anti-apoptotic effect was mentioned [60]. In glioma cells an inhibition of PKCδ by rottlerin also led to a reduced activity of ERK and Akt, and inhibited cell proliferation [61]. These findings show a strong cell specific effect of PKCδ. Our results suggest a proliferation promoting effect of PKCδ in renal carcinoma cells.
Conclusions
In conclusion, our results indicate that in CCF-RC1 and CCF-RC2 cells, PKCμ regulates tumor cell adhesion to umbilical vein endothelial cells. In CCF-RC1 cells, in addition to PKCμ, PKCε also regulates cell adhesion. Our observation of β1 integrin expression being regulated by PKCε, leads to the assumption that CCF-RC1 cell adhesion to endothelial cells is caused by affecting the expression of β1 integrins. In contrast, cell proliferation seems to be regulated by PKCδ.
Abbreviations
- BrdU:
-
bromodeoxyuridine
- CAMK:
-
Calcium/calmodulin-dependent protein kinase
- ECM:
-
extracellular matrix
- FAK:
-
focal adhesion dinase
- HUVEC:
-
human umbilical vein endothelial cell
- MTT:
-
3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide
- PBS:
-
phosphate buffered saline
- DNA-PK:
-
DNA dependent protein kinase
- PKC:
-
protein kinase C
- PKD:
-
protein kinase D
- RCC:
-
renal cell carcinoma.
References
Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991, 64: 327-336. 10.1016/0092-8674(91)90642-C.
Zernecke A, Weber KS, Erwig LP, Kluth DC, Schröppel B, Rees AJ, Weber C: Combinatorial model of chemokine involvement in glomerular monocyte recruitment: role of CXC chemokine receptor 2 in infiltration during nephrotoxic nephritis. J Immunol. 2001, 166: 5755-5762.
Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S: CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J Exp Med. 1992, 176: 245-253. 10.1084/jem.176.1.245.
McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA: Cell-cell interactions: leukocyte-endothelial interactions. Curr Opin Hematol. 2003, 10: 150-158. 10.1097/00062752-200303000-00009.
Greenwood J, Wang Y, Calder VL: Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. Immunology. 1995, 86: 408-415.
DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB: Immobilized stromal cell-derived factor-1alpha triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. J Immunol. 2007, 178: 3903-3911.
Mattila P, Majuri ML, Renkonen R: VLA-4 integrin on sarcoma cell lines recognizes endothelial VCAM-1. Differential regulation of the VLA-4 avidity on various sarcoma cell lines. Int J Cancer. 1992, 52: 918-923. 10.1002/ijc.2910520615.
Taichman DB, Cybulsky MI, Djaffar I, Longenecker BM, Teixidó J, Rice GE, Aruffo A, Bevilacqua MP: Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul. 1991, 2: 347-355.
Ruiz P, Dunon D, Sonnenberg A, Imhof BA: Suppression of mouse melanoma metastasis by EA-1, a monoclonal antibody specific for alpha 6 integrins. Cell Adhes Commun. 1993, 1: 67-81. 10.3109/15419069309095682.
Hangan D, Morris VL, Boeters L, von Ballestrem C, Uniyal S, Chan BM: An epitope on VLA-6 (alpha6beta1) integrin involved in migration but not adhesion is required for extravasation of murine melanoma B16F1 cells in liver. Cancer Res. 1997, 57: 3812-3817.
Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH: Involvement of integrin alpha(v)beta(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001, 12: 2699-2710.
Steinbach F, Tanabe K, Alexander J, Edinger M, Tubbs R, Brenner W, Stöckle M, Novick AC, Klein EA: The influence of cytokines on the adhesion of renal cancer cells to endothelium. J Urol. 1996, 155: 743-748. 10.1016/S0022-5347(01)66513-3.
Tomita Y, Saito T, Saito K, Oite T, Shimizu F, Sato S: Possible significance of VLA-4 (alpha 4 beta 1) for hematogenous metastasis of renal-cell cancer. Int J Cancer. 1995, 60: 753-758. 10.1002/ijc.2910600604.
Schwartz MA, Schaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu-Rev Cell Dev Biol. 1995, 11: 549-599. 10.1146/annurev.cb.11.110195.003001.
Kolanus W, Seed B: Integrins and inside-out signal transduction: converging signals from PKC and PIP3. Curr Opin Cell Biol. 1997, 9: 725-731. 10.1016/S0955-0674(97)80127-5.
Bouvard D, Molla A, Block MR: Calcium/calmodulin-dependent protein kinase II controls alpha5beta1 integrin-mediated inside-out signalling. J Cell Sci. 1998, 111: 657-665.
Huang CF, Fan JH, Chuang NN: Farnesyl pyrophosphate promotes and is essential for the binding of RACK1 With beta-tubulin. J Exp Zool. 2003, 298A: 119-127. 10.1002/jez.a.10277.
Rosfjord EC, Maemura M, Johnson MD, Torri JA, Akiyama SK, Woods VL, Dickson RB: Activation of protein kinase C by phorbol esters modulates alpha2beta1 integrin on MCF-7 breast cancer cells. Exp Cell Res. 1999, 248: 260-271. 10.1006/excr.1998.4390.
Brenner W, Benzing F, Gudejko-Thiel J, Fischer R, Färber G, Hengstler JG, Seliger B, Thüroff JW: Regulation of β1 integrin expression by PKCε in renal cancer cells. Int J Oncol. 2004, 25: 1157-1163.
Chun JS, Ha MJ, Jacobson BS: Differential translocation of protein kinase C epsilon during HeLa cell adhesion to a gelatin substratum. J Biol Chem. 1996, 271: 13008-13012. 10.1074/jbc.271.22.13008.
Azzi A, Boscoboinik D, Hensey C: The protein kinase C family. Eur J Biochem. 1992, 208: 547-557. 10.1111/j.1432-1033.1992.tb17219.x.
Valverde AM, Sinnett Smith J, Van Lint J, Rozengurt E: Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994, 91: 8572-8576. 10.1073/pnas.91.18.8572.
Hanks SK: Genomic analysis of the eukaryotic protein kinase superfamily: a perspective. Genome Biol. 2003, 4: 111.1-111.7. 10.1186/gb-2003-4-5-111.
Rozengurt E, Rey O, Waldron RT: Protein kinase D signaling. J Biol Chem. 2005, 280: 13205-13208. 10.1074/jbc.R500002200.
Jaken S: Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996, 8: 168-173. 10.1016/S0955-0674(96)80062-7.
Blobe GC, Obeid LM, Hannun YA: Regulation of protein kinase C and role in cancer biology. Cancer Metastasis Rev. 1994, 13: 411-431. 10.1007/BF00666107.
Brenner W, Färber G, Herget T, Wiesner C, Hengstler JG, Thüroff JW: Protein Kinase C eta is associated with progression of renal cell carcinoma (RCC): a systematic study of eleven PKC-isoforms in RCC in relation to histopathological parameters. Anticancer Res. 2003, 23: 4001-4004.
Manni A, Buckwalter E, Etindi R, Kunselman S, Rossini A, Mauger D, Dabbs D, Demers L: Induction of a less aggressive breast cancer phenotype by protein kinase C-alpha and -beta overexpression. Cell Growth Differ. 1996, 7: 1187-1198.
Borner C, Ueffing M, Jaken S, Parker PJ, Weinstein IB: Two closely related isoforms of protein kinase C produce reciprocal effects on the growth of rat fibroblasts. Possible molecular mechanisms. J Biol Chem. 1995, 270: 78-86. 10.1074/jbc.270.1.78.
Hashimura T, Tubbs RR, Connelly R, Caufield MJ, Trindade CS, McMahon JT, Galetti TP, Edinger M, Sandberg AA, Dal Cin P, Sait SJ, Pontes JE: Characterization of two cell lines with distinct phenotypes and genotypes established from a patient with renal cell carcinoma. Cancer Res. 1989, 49: 7064-7071.
Brenner W, Hempel G, Steinbach F, Hohenfellner R, Thüroff JW: Enhanced expression of ELAM-1 on endothelium of renal cell carcinoma compared to the corresponding normal renal tissue. Cancer Lett. 1999, 143: 14-21. 10.1016/S0304-3835(99)00172-X.
Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ: In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res. 2002, 64: 384-397. 10.1006/mvre.2002.2434.
Brenner W, Färber G, Herget T, Lehr HA, Hengstler JG, Thüroff JW: Loss of the tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer. 2002, 99: 53-57. 10.1002/ijc.10303.
Miles FL, Pruitt FL, van Golen KL, Cooper CR: Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008, 25: 305-324. 10.1007/s10585-007-9098-2.
Goekjian PG, Jirousek MR: Protein kinase C in the treatment of disease: signal transduction pathways, inhibitors, and agents in development. Curr Med Chem. 1999, 6: 877-903.
Brenner W, Greber I, Gudejko-Thiel J, Beitz S, Schneider E, Walenta S, Peters K, Unger R, Thüroff JW: Migration of renal carcinoma cells is dependent on protein kinase Cdelta via beta1 integrin and focal adhesion kinase. Int J Oncol. 2008, 32: 1125-1131.
Chen J, Deng F, Singh SV, Wang QJ: Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res. 2008, 68: 3844-3853. 10.1158/0008-5472.CAN-07-5156.
Van Lint J, Rykx A, Vantus T, Vandenheede JR: Molecules in focus: Getting to know protein kinase D. Int J Biochem Cell Biol. 2002, 34: 577-581. 10.1016/S1357-2725(01)00163-7.
Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC: An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999, 18: 4440-4449. 10.1038/sj.onc.1202827.
De Kimpe L, Janssens K, Derua R, Armacki M, Goicoechea S, Otey C, Waelkens E, Vandoninck S, Vandenheede JR, Seufferlein T, Van Lint J: Characterization of cortactin as an in vivo protein kinase D substrate: interdependence of sites and potentiation by Src. Cell Signal. 2009, 21: 253-263. 10.1016/j.cellsig.2008.10.015.
Eiseler T, Döppler H, Yan IK, Goodison S, Storz P: Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009, 11: R13-10.1186/bcr2232.
Syed V, Mak P, Du C, Balaji KC: β-Catenin mediates alteration in cell proliferation, motility and invasion of prostate cancer cells by differential expression of E-cadherin and protein kinase D1. J Cell Biochem. 2008, 104: 82-95. 10.1002/jcb.21603.
Du C, Jaggi M, Zhang C, Balaji KC: Protein kinase D1-mediated phosphorylation and subcellular localization of beta-catenin. Cancer Res. 2009, 69: 1117-1124. 10.1158/0008-5472.CAN-07-6270.
Woods AJ, White DP, Caswell PT, Norman JC: PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004, 23: 2531-2543. 10.1038/sj.emboj.7600267.
Higai K, Miyazaki N, Azuma Y, Matsumoto K: Interleukin-1beta induces sialyl Lewis × on hepatocellular carcinoma HuH-7 cells via enhanced expression of ST3Gal IV and FUT VI gene. FEBS Lett. 2006, 580: 6069-6075. 10.1016/j.febslet.2006.09.073.
Cowell CF, Yan IK, Eiseler T, Leightner AC, Döppler H, Storz P: Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J Cell Biochem. 2009, 106: 714-728. 10.1002/jcb.22067.
Steinbach F, Tanabe K, Alexander J, Edinger M, Tubbs R, Brenner W, Stöckle M, Novick AC, Klein EA: The influence of cytokines on the adhesion of renal cancer cells to endothelium. J Urol. 1996, 155: 743-748. 10.1016/S0022-5347(01)66513-3.
Ivaska J, Whelan RD, Watson R, Parker PJ: PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002, 21: 3608-3619. 10.1093/emboj/cdf371.
Palmantier R, George MD, Akiyama SK, Wolber FM, Olden K, Roberts JD: Cis-polyunsaturated fatty acids stimulate beta1 integrin-mediated adhesion of human breast carcinoma cells to type IV collagen by activating protein kinases C-epsilon and -mu. Cancer Res. 2001, 61: 2445-2452.
Engers R, Mrzyk S, Springer E, Fabbro D, Weissgerber G, Gernharz CD, Gabbert HE: Protein kinase C in human renal cell carcinomas: role in invasion and differential isoenzyme expression. Br J Cancer. 2000, 82: 1063-1069. 10.1054/bjoc.1999.1043.
Petiti JP, De Paul AL, Gutiérrez S, Palmeri CM, Mukdsi JH, Torres AI: Activation of PKC epsilon induces lactotroph proliferation through ERK1/2 in response to phorbol ester. Mol Cell Endocrinol. 2008, 289: 77-84. 10.1016/j.mce.2008.04.015.
Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E: Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in swiss 3T3 cells. J Biol Chem. 2004, 279: 16883-16893. 10.1074/jbc.M313225200.
Brändlin I, Hübner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, Link G, Rupp S, Storz P, Pfizenmaier K, Johannes FJ: Protein kinase C (PKC)eta-mediated PKC mu activation modulates ERK and JNK signal pathways. J Biol Chem. 2002, 277: 6490-6496. 10.1074/jbc.M106083200.
Brenner W, Wiesner C, Färber G, Herget T, Hengstler JG, Thüroff JW: Gender specific expression of tumor suppressor PKCδ versus oncogenic PKCη in renal cell carcinoma. EXCLI Journal. 2003, 2: 45-51.
Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y: Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc Natl Acad Sci USA. 1992, 89: 10159-10163. 10.1073/pnas.89.21.10159.
Ashton AW, Watanabe G, Albanese C, Harrington EO, Ware JA, Pestell RG: Protein kinase Cdelta inhibition of S-phase transition in capillary endothelial cells involves the cyclin-dependent kinase inhibitor p27(Kip1). J Biol Chem. 1999, 274: 20805-20811. 10.1074/jbc.274.30.20805.
Fukumoto S, Nishizawa Y, Hosoi M, Koyama H, Yamakawa K, Ohno S, Morii H: Protein kinase C delta inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997, 272: 13816-13822. 10.1074/jbc.272.21.13816.
Bharti A, Kraeft SK, Gounder M, Pandey P, Jin S, Yuan ZM, Lees-Miller SP, Weichselbaum R, Weaver D, Chen LB, Kufe D, Kharbanda S: Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Mol Cell Biol. 1998, 18: 6719-6728.
Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O: The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase Cdelta. Blood. 1997, 90: 4341-4353.
Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T: Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002, 100: 3741-3748. 10.1182/blood-2002-02-0539.
Jane EP, Premkumar DR, Pollack IF: Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther. 2006, 319: 1070-1080. 10.1124/jpet.106.108621.
Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F: Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994, 199: 93-98. 10.1006/bbrc.1994.1199.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/10/183/prepub
Acknowledgements
This work was supported by the Priority Program Minimal Invasive Surgery.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WB: study design, interpretation of the results, drafting of the manuscript. SB: cell adhesion experiments. ES: cell migration experiments. FB: flow cytometry analyses. REU: participation in flow cytometry analyses, interpretation of the results, critical revision of the manuscript. FR: participation in the study design, critical revision of the manuscript. JWT: participation in the study design, critical revision of the manuscript. CH: participation in the study design, critical revision of the manuscript
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Brenner, W., Beitz, S., Schneider, E. et al. Adhesion of renal carcinoma cells to endothelial cells depends on PKCμ. BMC Cancer 10, 183 (2010). https://doi.org/10.1186/1471-2407-10-183
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-10-183