Abstract
Background
Normal and abnormal processes of pregnancy and childbirth are poorly understood. This second article in a global report explains what is known about the etiologies of preterm births and stillbirths and identifies critical gaps in knowledge. Two important concepts emerge: the continuum of pregnancy, beginning at implantation and ending with uterine involution following birth; and the multifactorial etiologies of preterm birth and stillbirth. Improved tools and data will enable discovery scientists to identify causal pathways and cost-effective interventions.
Pregnancy and parturition continuum
The biological process of pregnancy and childbirth begins with implantation and, after birth, ends with the return of the uterus to its previous state. The majority of pregnancy is characterized by rapid uterine and fetal growth without contractions. Yet most research has addressed only uterine stimulation (labor) that accounts for <0.5% of pregnancy.
Etiologies
The etiologies of preterm birth and stillbirth differ by gestational age, genetics, and environmental factors. Approximately 30% of all preterm births are indicated for either maternal or fetal complications, such as maternal illness or fetal growth restriction. Commonly recognized pathways leading to preterm birth occur most often during the gestational ages indicated: (1) inflammation caused by infection (22-32 weeks); (2) decidual hemorrhage caused by uteroplacental thrombosis (early or late preterm birth); (3) stress (32-36 weeks); and (4) uterine overdistention, often caused by multiple fetuses (32-36 weeks). Other contributors include cervical insufficiency, smoking, and systemic infections. Many stillbirths have similar causes and mechanisms. About two-thirds of late fetal deaths occur during the antepartum period; the other third occur during childbirth. Intrapartum asphyxia is a leading cause of stillbirths in low- and middle-income countries.
Recommendations
Utilizing new systems biology tools, opportunities now exist for researchers to investigate various pathways important to normal and abnormal pregnancies. Improved access to quality data and biological specimens are critical to advancing discovery science. Phenotypes, standardized definitions, and uniform criteria for assessing preterm birth and stillbirth outcomes are other immediate research needs.
Conclusion
Preterm birth and stillbirth have multifactorial etiologies. More resources must be directed toward accelerating our understanding of these complex processes, and identifying upstream and cost-effective solutions that will improve these pregnancy outcomes.
Similar content being viewed by others
Background
As succinctly stated by Romero et al., "Few biological processes as central to the survival of a species as parturition are so incompletely understood" [1]. This is especially relevant for understanding mechanisms associated with preterm birth and stillbirth. Unfortunately, this lack of knowledge about the process of giving birth has led to largely empirical and ineffective interventions.
Two compelling principles emerge from the current understanding of pregnancy and parturition. First, labor represents a natural continuum of processes that begin at implantation and culminate with the return of the uterus to its non-pregnant state [2, 3]. Parturition proceeds through well-defined phases (Figure 1):
-
Implantation of the blastocyst within the endometrium and characterized by embryonic trophoblast invasion of maternal spiral arteries, allowing establishment of placentation
-
Uterine quiescence, during which embryogenesis and fetal growth occur and the uterus increases dramatically in size through hypertrophy
-
Activation of the myometrium, during which cellular and biochemical events occur that promote uterine contractility
-
Stimulation, or the onset of regular uterine contractions characteristic of labor and birth
-
Involution, during which the uterus reduces in size and returns to its non-pregnant state—abnormalities in uterine involution are associated with maternal postpartum hemorrhage, a leading cause of maternal mortality globally
The overwhelming majority of pregnancy is spent in uterine quiescence or in activation. Less than 0.5% of pregnancy is spent in active labor, yet most interventions and research have focused on treatment of preterm labor or other intrapartum events. As noted below, it is likely that research directed at understanding the mechanisms maintaining uterine quiescence and the mechanisms of activation that allow the uterus to contract will have significant impact upon the development of rational and efficacious prevention strategies.
The second compelling principle is that preterm birth and stillbirth are complex outcomes with multifactorial etiologies. Preterm birth and stillbirth represent final common outcomes from a wide variety of causes, each with distinct biologic pathways [4, 5]. Unfortunately, all preterm births or stillbirths have usually been defined as a single endpoint, regardless of etiology, for epidemiological purposes. This has led to uniform and largely unsuccessful treatments or interventions. In fact, the etiologies of preterm births and stillbirths differ according to gestational age, ethnicity, and characteristics unique to each population. For example, intrauterine infection and decidual hemorrhage are important causes of extreme prematurity and of stillbirth [6], while uterine overdistension associated with multiple gestations and maternal or fetal stress are important causes of preterm birth from 32 to 36 weeks of gestation.
Regardless of initiating factors, these pathways ultimately lead to activation of the fetal membranes and maternal decidua, resulting in common mediators such as prostaglandins and metalloproteinases that stimulate contractility and rupture of the fetal membranes. The finding that heterogeneous origins result in common downstream biological pathways and outcomes provides the opportunity to develop rational treatment strategies that target the unique upstream initiating event and the common downstream effectors [4].
Finally, disparities exist in medically-indicated preterm birth secondary to maternal illness or fetal compromise. In high-income countries, the greatest increases in preterm birth rates have occurred among late preterm births from 34 to 37 weeks of gestation, which now account for 60%-70% of all preterm births [7]. Much of the increase in late preterm births has been attributed to the increased prevalence of multiple gestations associated with assisted reproductive technologies and with medically-indicated preterm birth. Medically-indicated preterm birth is the most rapidly increasing cause of preterm birth in high-income countries, responsible for 30% of all preterm births [7]. In the United States, medically indicated preterm birth increased by 55% from 1989 through 2000 [8]. However, in resource-poor low- and middle-income countries where fetal and maternal well-being assessment technology is not available, medically-indicated preterm birth accounts for less than 10% of preterm births. One consequence of this disparity has been a reduction in stillbirths and perinatal mortality, but a paradoxical increase in preterm births among high-income countries.
Events characterizing both spontaneous parturition and preterm parturition, and critical gaps in the understanding of these processes are described in the following sections.
Spontaneous term parturition
Understanding events surrounding normal parturition at term is necessary for an informed discussion of pathologic mechanisms leading to preterm parturition. Following implantation, the process of normal spontaneous parturition can be divided into the phases described below in Figure 1(modified from [3, 9]).
Pregnancy proceeds through a series of orderly phases, beginning with implantation of the early blastocyst and culminating with delivery and return of the uterus to the non-pregnant state. Perturbations in any of these steps may cause adverse pregnancy outcomes.
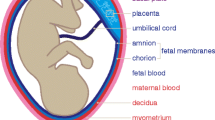
Implantation
Implantation is characterized by embryonic cytotrophoblast invasion of the endometrium and maternal spiral arteries and is necessary to establish normal placentation. This process is facilitated by maternal immunologic processes that involve uterine natural killer monocytes (NK cells) and a shift from a pro-inflammatory to an anti-inflammatory intrauterine milieu. Perturbations in implantation have been associated with habitual abortions, stillbirths, and with preeclampsia, which is an important cause of medically-indicated preterm birth [10].
Phase 0: Quiescence
Throughout the majority of pregnancy the uterus remains relaxed and quiescent. Myometrial activity is inhibited by a variety of biologic compounds including progesterone, nitric oxide, and relaxin. Rare uterine contractions during the quiescent phase are of low frequency and amplitude and are poorly coordinated throughout the uterus; these are commonly referred to as Braxton-Hicks contractions in women. The poor coordination of these contractions is due to an absence of gap junctions and contractile-associated proteins that otherwise allow direct cell-to-cell coupling of electrical signaling [11].
Phase 1: activation
Myometrial activation occurs in Phase 1 and is characterized by increased expression of myometrial contractile-associated proteins and cellular receptors for oxytocin and prostaglandins, both of which stimulate uterine contractility [12]. The signals for myometrial activation come from uterine stretch (which induces contractile-associated protein and oxytocin gene expression) and from maturation of the fetal hypothalamic-pituitary-adrenal (HPA) axis, as characterized below.
Phase 2: stimulation
Phase 2 is a progressive cascade of events leading to a common pathway of parturition involving high amplitude, regular uterine contractions, cervical ripening, and activation of the decidua and fetal membranes. Estrogens, produced by the placenta play a central role in the coordination of these events. Estrogens increase the expression of many contraction-associated proteins, including connexin 43, and of oxytocin and prostaglandin receptors; estrogens also promote prostaglandin production and increases myosin light-chain kinase (MLCK, which stimulates myometrial contractions) [3]. These changes promote myometrial contractility. Additionally, there is a shift in progesterone receptor (PR) isoforms from the normally dominant PR-B to a truncated, inactive PR-A, leading to a state of functional progesterone withdrawal that promotes myometrial contractility. Placental estrogen production is dependent upon precursor fetal adrenal androgens that are aromatized by placental steroid aromatase into estrogens. Thus, the events of Phase 2 are characterized by maturation and activation of the fetal HPA axis. The events leading to fetal HPA activation are incompletely understood but it is thought that placental corticotrophin-releasing hormone (CRH) plays a central role [13]. CRH, a neuropeptide of predominantly hypothalamic origin, is also expressed in the human placenta and membranes and released in exponentially increasing amounts over the course of gestation into maternal and fetal compartments. The trajectory of CRH rise has been associated with the length of gestation [14]. These findings have led some researchers to suggest that placental CRH may act as a "placental clock" and regulate the length of gestation [14]. Placental CRH synthesis is stimulated by adrenal glucocorticoids. Placental CRH, in turn, promotes fetal cortisol and androgen production, and this positive feedback loop is progressively amplified, thereby driving the process forward from fetal HPA activation to estrogen biosynthesis and parturition.
Cervical softening and decidual and fetal membrane activation also occur during Phase 2. Cervical ripening is characterized by a decrease in total collagen content, an increase in collagen solubility, and an increase in collagenolytic activity that results in the remodeling of the extracellular matrix of the cervix [15]. Prostaglandins, estrogens, progesterones, and inflammatory cytokines all promote extracellular matrix metabolism and cervical ripening. Decidual and fetal membrane activation refers to a complex set of anatomical and biochemical events eventually resulting in the rupture of membranes. The precise mechanism of the decidual and fetal membrane activation is not yet known, but extracellular matrix-degrading enzymes such as matrix metalloproteinase 1 (MMP-1), interstitial collagenase, MMP-8 (neutrophil collagenase), MMP-9 (gelatinase-B), neutrophil elastase, and plasmin have been implicated. These enzymes degrade extracellular matrix proteins (e.g., collagens and fibronectins), thereby weakening the membranes and eventually leading to the rupture of membranes.
Phase 3: involution
Phase 3 begins with the third stage of labor and involves placental separation and uterine contraction. Placental separation occurs by cleavage along the plane of the decidua basalis. Uterine contraction is essential to prevent bleeding from large venous sinuses that are exposed after delivery of the placenta, and is primarily affected by oxytocin. Postpartum hemorrhage, an abnormality of Phase 3, is a leading cause of maternal mortality worldwide.
Summary of spontaneous term parturition
Parturition involves a progressive cascade of events initiated by HPA activation and increased placental CRH expression, leading to a functional progesterone withdrawal and estrogen activation, which results in the expression and activation of contraction-associated proteins (CAPs), including oxytocin, and prostaglandin receptors. This biological cascade eventually leads to a common pathway involving cervical ripening, uterine contractility, decidual and fetal membrane activation, and, in the second stage, increases in maternal oxytocin. It has been hypothesized that both preterm and term labor share this common pathway and that pathological stimuli of parturition, as described in the following sections, may act in concert with the normal physiological preparation for labor, especially after 32 weeks of gestation. Prior to 32 weeks of gestation, a greater degree of pathological stimulus may be required to initiate labor.
One fundamental difference between the spontaneous parturition at term and preterm labor is that term labor results from physiological activation of components of the common pathway, while preterm labor arises from pathological processes that activate one or more of the components of the common pathway of parturition. However, further research is necessary to answer fundamental questions, including: 1. What are the mechanisms that maintain uterine quiescence for greater than 95% of the total length of gestation? 2. What is the basis for the disparities in gestational length and risks of preterm birth between ethnic and socioeconomic groups? 3. Do they have a biologic basis, or can they be accounted for by environmental factors?
Pathways to spontaneous preterm birth
Preterm birth may result from preterm labor with intact fetal membranes, preterm rupture of the fetal membranes, or from iatrogenic preterm delivery for maternal or fetal indications. In high-income countries, approximately 40-45% of preterm births follow preterm labor, 25-40% follow preterm premature rupture of the fetal membranes, and 30-35% are indicated deliveries [7]. In contrast, studies from countries in Latin America have shown that almost 70% are spontaneous preterm births, 16-21% involve rupture of membranes, and 11-15% have medically induced causes [16, 17].
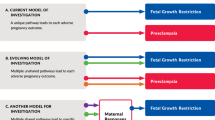
Until recently, a tendency has existed among obstetricians and epidemiologists to combine, for statistical purposes, all preterm births occurring between 22 and 37 weeks of gestation. The traditional empirical approach to preterm labor presupposed a single pathologic process for which treatment could be uniform. It is now clear the causes of preterm labor are multifactorial and vary according to gestational age, genetic, and environmental factors. A useful paradigm for pathologic pathways contributing to preterm birth has been provided by Lockwood and Kuczynski [18]. These pathways include systemic and intrauterine infection, uteroplacental thrombosis and intrauterine vascular lesions or decidual hemorrhage, stress, and uterine overdistension (Table 1). While each of these may cause preterm birth at any point in gestation, infection/inflammation predominates as a cause of early preterm birth (24-32 weeks gestation), and stress and uterine overdistension are associated mostly with late preterm birth (32-37 weeks).
Additionally, each pathway may be influenced by gene-environment interactions and by genetic variability in single nucleotide polymorphisms, described below. Commonly occurring pathways of preterm parturition are described below.
Infection/inflammation
Infections are strongly related to preterm birth [19]. Infectious sources linked to preterm birth include intrauterine infections, lower genital tract infections, and systemic maternal infections.
Intrauterine infection is recognized as one of the most important and potentially preventable causes of early preterm birth. These infections are thought to be responsible for up to 50% of extreme preterm births of less than 28 weeks of gestation, where both neonatal mortality and morbidity are high. The prevalence of microbial invasion of the chorioamnion is 73% in women with a spontaneous preterm birth prior to 30 weeks of gestation, and only 16% among women with indicated preterm delivery without labor [20]. One sentinel study found the frequency of intrauterine infection with recovery of micro-organisms from amniotic fluid to be 45% at 23-26 weeks of gestation, 16% at 27-30 weeks, and 11% at 31-34 weeks [21]. Intrauterine infection as a cause of preterm birth is rare beyond 34 weeks of gestation. It is likely that recent microbiology techniques such polymerase chain reaction identification of fastidious microorganisms will lead to even greater estimates of infection as a cause of preterm birth.
Furthermore, a high proportion of women in preterm labor with evidence of microbial invasion of the amniotic fluid are refractory to standard tocolytic therapy and experience rapid preterm delivery (62% versus 13% of women with sterile amniotic fluid) [22]. This suggests the pathophysiology of infection-associated preterm labor differs from that of idiopathic preterm labor. There is now considerable evidence to suggest the pro-inflammatory cytokine/prostaglandin cascade plays a central role in the pathogenesis of infection-associated preterm birth [23]. These inflammatory mediators are produced by macrophages, decidual cells, and fetal membranes in response to bacteria or bacterial products. A role for selected cytokines in preterm labor is based upon the following observations: elevated amniotic fluid concentrations of cytokines and prostaglandins are found in patients with intra-amniotic infection and preterm labor; in-vitro, bacterial products stimulate production of pro-inflammatory cytokines by human decidua; these cyto- kines, in turn, stimulate production of prostaglandins by amnion and decidua; administration of interleukin-1 to pregnant mice or non-human primates induces preterm labor which can be prevented by administration of Il-1 receptor antagonist protein.
There is also evidence that lower genital tract infections, especially bacterial vaginosis, or maternal systemic infections such as periodontitis, malaria, and syphilis contribute to preterm birth (see article 3 on interventions), as briefly reviewed below relevant to pathophysiology.
Bacterial vaginosis and periodontitis
Observational studies show increased risks of intra-amniotic infection, preterm delivery delivery, low birth weight, and endomyometritis in the presence of bacterial vaginosis (BV) [24, 25]. Generally, the presence of BV increases the risk of preterm birth by 50%. However, most women with BV do not have a preterm birth and randomized clinical trials assessing treatment of BV for the prevention of preterm delivery have been disappointing, although treatment of women prior to 20 weeks may be beneficial [26]. These are reviewed in article 3.
Similarly, observational studies have suggested an association between preterm birth or other adverse pregnancy outcomes and periodontal disease [27]. However, a recent meta-analysis of 44 studies, including five controlled trials, found no association between periodontal disease and preterm birth although low- income countries had higher trends towards association [28]. Heterogeneity between studies was noted, such as inconsistent disease and outcome definitions, inadequate control for confounders and insufficient sample size, making firm conclusions difficult to make [28].
The associations between BV or periodontal disease and preterm birth, and the reasons for discrepancy in treatment trials are important to understand, since both BV and periodontal disease occur commonly in pregnancy. Bacterial vaginosis is seen in up to 30% of pregnant women [24]. In high-income countries it is estimated that about 15% of adults between 21-50 years have severe periodontitis [29]. The proportion may be even higher in low-income-countries. Explanations for these discrepancies may include (1) gestational age at diagnosis/ enrollment; (2) past reproductive history; and (3) choice of treatment. Recent research indicates that both BV and periodontitis are complex microbiological processes, involving a wide variety of both cultivable and fastidious microorganisms not previously identified that may be a part of the normal microflora [30, 31]. Further, both BV and periodontal are associated with perturbations in the host inflammatory response, characterized by a pro-inflammatory state. These differences have led some to hypothesize that only a subgroup of women harboring certain microbes associated with BV or periodontal disease, or with genetic differences in inflammatory responsiveness may be at risk for preterm birth. These women may have an abnormal inflammatory response to changes in the vaginal ecosystem (either hypo- or hyper- responsive) predisposing them to preterm delivery . In support of this concept, Macones, et al, recently reported that women with the polymorphism coding for TNFα-308 allele that leads to up-regulation of TNF-α are at increased risk of preterm delivery associated with BV [32]. An understanding of the relationships among the human microbiome, host inflammatory responsiveness and pregnancy outcome represents a critical research need.
Malaria
Observational studies have demonstrated an association between malaria and risk of preterm birth with odds ratios ranging between 2 and 3 [33–36], although most randomized trials of malaria prevention or treatment do not report specifically on preterm births. However, the evidence of impact on low birth weight is strong, as reviewed in article 3. The magnitude of effect on preterm delivery appears to be based on a number of factors, including timing of infection [33, 37], underlying parity [38], severity of infection [33], increasing placental parasitemia [39], and local transmission rates [35]. Placental malaria is also significantly associated with a higher risk for stillbirth (see article 3 on interventions).
Erythrocytes infected with P. falciparum differ in important ways from non-pregnant individuals. Placental sequestration occurs in the intervillous space. Placental infection generates an immune response that is characterized by monocytic infiltrates in the placental intervillous space and a change in cytokine balance from Th-2 to Th-1 (specifically increased levels of TNF-α, INF-y, and IL-1P). Elevated levels of these cytokines, particularly TNF-α, have been associated with low birth weight and preterm delivery [40].
Syphilis
Syphilis, caused by Treponema pallidum, is a risk factor for both stillbirth and preterm birth (see article 3 on interventions). It is estimated to be responsible for 460,000 abortions or stillbirths per year with higher rates in low-income countries [41]. The study of T. pallidum is hindered by the fact that it cannot be cultivated for sustained periods using artificial media. However, the complete genome sequence of T. pallidum has been published within the past 10 years and provides opportunities for new insight into its pathogenesis. Little is known about its mechanism of action or determinants of virulence [42]. Treponema are able to cross the placenta and cause infection in the fetus [43]. Primary and secondary syphilis in pregnancy will lead to fetal infection in virtually all cases, with approximately 30-50% of pregnancies resulting in stillbirth or death shortly after delivery [43]. In contrast, fetal infection is less common with tertiary syphilis. The immune response to T. pallidum in pregnancy has not been vigorously studied, but the dampening of the intensity of the innate and cell-mediated immune response to favor the maintenance of the fetus may result in incomplete clearance and the development of chronic infection and adverse pregnancy outcome [43]. However, the nature and role of the maternal-fetal immune response, their interactions, and effect on pregnancy outcome remains poorly understood [44].
Decidual hemorrhage/thrombosis
Decidual hemorrhage may cause either late or early preterm birth. Vascular lesions of the placenta are commonly associated with preterm birth and preterm premature rupture of membranes (PPROM). Vascular lesions of the placenta have been reported in 34% of women with preterm delivery, 35% of women with PPROM, and in 12% of term uncomplicated deliveries [45]. These lesions may be characterized as failure of physiologic transformation of the spiral arteries, atherosis, and maternal or fetal arterial thrombosis. The proposed mechanism linking vascular lesions to preterm birth is related to utero-placental ischemia. Although the pathophysiology remains unclear, thrombin is thought to play a central role.
Independent of its critical role in coagulation, thrombin is a multifunctional protease that elicits contractile activity of vascular, intestinal, and myometrial smooth muscle. Thrombin stimulates increases in basal tone and phasic contractions in longitudinal myometrial smooth muscle in vitro, in a dose-dependent manner [46]. Recently, these in vitro observations have been confirmed with an in vivo model utilizing thrombin and myometrial contractility could be significantly reduced by the addition of heparin, a known thrombin inhibitor. These in vitro and in vivo experiments provide a possible mechanistic explanation for increased uterine activity clinically observed in abruption placentae and preterm birth following first or second trimester bleeding.
A relationship between thrombin and PPROM may also exist. Matrix metalloproteinases (MMPs) break down the extracellular matrix of the fetal membranes and the choriodecidua and contribute to PPROM, as discussed below. In vitro, thrombin significantly increases MMP-1, MMP-3, and MMP-9 protein expression in decidual cells and fetal membranes collected from uncomplicated term pregnancies [47–49]. Thrombin also elicits a dose- dependent increase in decidual interleukin-8, a chemo- attractant cytokine responsible for neutrophil recruitment [50]. Overt placental abruption, an extreme example of decidual hemorrhage, is also associated with a marked decidual infiltration of neutrophils, a rich source of proteases and matrix metalloproteinases [50]. This may provide a mechanism for premature rupture of the membranes (PROM) in the setting of decidual hemorrhage.
Maternal or fetal HPA activation: stress
Stress results in preterm activation of the maternal or fetal HPA axis and is increasingly recognized as an important cause of late preterm birth. Stress may be simply defined as any challenge, whether physical or psychological, that threatens or is perceived to threaten homeostasis of the patient. Several studies have found 50% to 100% increases in preterm birth rates associated with maternal stress, usually defined as a composite of life events, anxiety, depression, or perceived stress [51]. Neuroendocrine, immune, and behavioral processes (such as depression) have been linked to stress-related preterm birth. However, the most important processes linking stress and preterm birth are neuroendocrine, resulting in preterm activation of the HPA axis. These processes are mediated by placental CRH (reviewed in [52]). In vitro studies of human placental cells have shown CRH is released from cultured human placental cells in a dose-response manner to all major biological effectors of stress, including cortisol, catecholamines, oxytocin, angiotension II, and interleukin-1. In vivo studies have also found significant correlations between maternal psychosocial stress and maternal plasma levels of CRH, ACTH, and cortisol. Several studies have related early maternal plasma CRH to the timing of birth. Hobel and colleagues [53] conducted serial assessments of CRH over the course of gestation and found that compared with women delivering at term, women delivering preterm had significantly elevated CRH levels as well as a significantly accelerated rate of CRH increase over the course of their gestation. In addition, they found that maternal psychosocial stress levels at mid-gestation significantly predicted the magnitude of increase in maternal CRH levels between mid-and later-gestation.
These data suggest the relationship between maternal psychological stress and prematurity may be mediated by prematurely increased expression of placental CRH. In term parturition, placental CRH activation is driven largely by the fetal HPA axis in a forward feedback loop upon fetal maturation. In preterm parturition the maternal HPA axis may drive placental CRH expression [52]. Maternal stress, in the absence of other mediators of preterm birth such as infection, results in increased levels of biological effectors of stress including cortisol and epinephrine, which could activate placental CRH gene expression. Placental CRH, in turn, can stimulate fetal secretion of cortisol and DHEA-S (via activation of fetal HPA axis) and placental synthesis of estrogens and prostaglandins, thereby precipitating preterm delivery (reviewed in [54]). Stress may contribute to the increased rates of preterm birth observed among African-Americans in the United States.
Asphyxia may represent a common final outcome for a variety of pathways that include stress, hemorrhage, preeclampsia, and infection. Asphyxia plays an important role in preterm birth, stillbirth, and adverse neonatal developmental sequelae. Chronic asphyxia—associated with insufficiency of the utero-placental circulation— may occur in placental infection such as malaria, or maternal illnesses (e.g., diabetes, preeclampsia, chronic hypertension), and is characterized by activation of the fetal HPA axis and subsequent preterm delivery.
Uterine overdistension
Uterine overdistension plays a key role in the onset of preterm labor associated with multiple gestations, polyhydramnios, and macrosomia. Multiple gestations, frequently attributable to assisted reproduction technologies (ART) including ovulation induction and in-vitro fertilization, are one of the most important causes of late preterm birth in high-income countries (HICs). In the United States, for example, ART accounts for 1% of all live births, but 17% of all multiple births; 53% of neonates conceived as a result of ART in 2003 were multiples [4]. The mechanisms whereby uterine overdistension might lead to preterm labor are incompletely understood, and appropriate animal models are lacking. Uterine stretch induces expression contraction-associated proteins such as oxytocin receptors and connexin-43 [55, 56]. In vitro stretch of myometrial strips also increases prostaglandin H synthase 2 (PGHS-2) and prostaglandin E [57]. Stretching the muscle of the lower uterine segment has been shown to increase interleukin-8 (IL-8) and collagenase production, which in turn facilitates cervical ripening [58, 59]. However, experimental animal models for uterine overdistension do not currently exist, and human studies have been entirely observational in nature. This is a critical gap in our understanding, given the increasing prevalence of multiple gestations in HICs.
Cervical insufficiency
Cervical insufficiency has traditionally been associated with second trimester pregnancy losses, but recent evidence suggests that cervical disorders are associated with a wide variety of adverse pregnancy outcomes, including early preterm birth [60–62]. Cervical insufficiency has traditionally been identified among women with a history of recurrent mid-trimester pregnancy losses in the absence of recognized uterine contractions. There are five recognized or possible causes: (1) congenital disorders; (2) in-utero diethylstilbestrol exposure (3) loss of cervical tissue following a surgical procedure such as Loop Electrosurgical Excision Procedure (LEEP) or conization; (4) traumatic damage; and (5) infection.
Traditionally, women with a history of cervical insufficiency were offered cervical cerclage in early pregnancy. However, it has been proposed, and it is most likely, that most cases of cervical insufficiency represent a continuum of premature tissue remodeling and cervical shortening from other pathological processes for which cerclage may not always be appropriate and are better predicted by cervical length determined by transvaginal ultrasonography [60]. Cervical length measured by transvaginal ultrasound is inversely correlated with risk of preterm birth [61]; 50% of women with a cervical length of 15 millimeters or less at 22-24 weeks deliver prior to 32 weeks of gestation [63]. Further, there is a correlation between the length of a previous gestation resulting in preterm birth and cervical length in the next pregnancy, but no correlation with an obstetrical history of cervical insufficiency and cervical length in the next pregnancy [60].
These data suggest that the true cervical insufficiency occurs infrequently, and a short cervix more frequently occurs as a consequence of premature cervical remodeling as a result of a pathological process. Infection and inflammation likely play a significant role in cervical shortening and premature dilation. Fifty percent of patients evaluated by amniocentesis for mid-trimester asymptomatic cervical dilation and 9% of patients with a cervical length of <25 millimeters but without cervical dilation have evidence of intra-amniotic infection[64, 65]. These data suggest an important role for ascending intrauterine infection, as outlined above, in the short cervix and cervical insufficiency.
Preterm premature rupture of the fetal membranes
Regardless of the etiology or mechanistic pathway to spontaneous preterm labor, preterm birth is usually preceded by preterm premature rupture of the fetal membranes. Preterm premature rupture of membranes (PPROM) accounts for 25-40% of preterm births [7, 66] and represents a final common pathway to preterm birth. Thus, an understanding of the mechanistic pathways leading to PPROM is important in understanding the biologic basis of prematurity. Preterm pre-labor rupture of membranes has been associated with intrauterine infection, tobacco use, abruption, multiple gestations, previous PPROM, previous cervical surgery or laceration, a short cervix by ultrasound, genetic connective tissue disorders, and vitamin C deficiency.
Collagen provides the major structural strength for the fetal membranes. Loss of collagen and structural strength leading to rupture of the membranes is mediated by matrix metalloproteinases (MMPs), a large family of enzymes that act to degrade collagen and remodel tissue. Matrix metalloproteinase types 1 and 8 are collagenases that act to degrade collagen types I, II, and III. The activity of MMPs is regulated at several levels, but most importantly by tissue inhibitors of MMPs (TIMPs). A balance between activators and tissue inhibitors of metalloproteinases controls metalloprotease activity. An increased ratio of MMP 9 to TIMP 1 is associated with decrease tensile strength of fetal membranes (reviewed in [67]. MMPs 1-3, 8, 9 and 14 are upregulated in the amnion and chorion and their concentrations increased in amniotic fluid in PPROM [67]. Amniotic fluid MMP 9 concentrations are increased in the amniotic fluid of patients with PPROM, and to a lesser extent in preterm birth [68]. Increased pro-MMP 9 is found overlying the cervix in term gestation.
The activity of MMP's is primarily upregulated by decidual/fetal membrane activation during Phase 2 of spontaneous parturition at term. However, in pathologic conditions associated with preterm birth, MMP production is stimulated by infection, inflammation, or decidual hemorrhage. Bacteria or bacterial products directly secrete collagenases or stimulate MMP production [69]. Pro-inflammatory cytokines such as IL-1 and TNF-alpha also increase MMP production and decrease TIMPs in cultured membranes [70]. Thrombin, generated as a result of choriodecidual hemorrhage, also increases MMP-9 production in amniochorion cultures [49]. Finally, stretching of the membranes by uterine overdistension may result in PROM by increasing interleukin-8 and MMP activity [58].
Genetics, environment and gene-environment interactions
There is compelling evidence that a genetic predisposition to preterm birth exists [71, 72]. The evidence for a genetic or epigenetic contribution to preterm birth includes: 1. twin studies that demonstrate heritability; 2. increased recurrence risks for preterm birth among women with prior preterm birth; 3. increased risks of having preterm birth for women who were themselves born preterm; 4. increased risks of preterm birth for sisters of women who have had a preterm birth; and 5. racial disparities in preterm birth that are independent of socioeconomic factors (see [71] for a review). Twin studies have indicated a heritability of 25-40% for preterm birth [73].
Genome-wide association studies, however, have failed to identify consistent candidate genes for preterm birth. This is likely due to genetic heterogeneity. Preterm birth is a complex phenotype, with many etiologic and pathophysiological pathways that likely cannot be explained by genetic variation alone. An alternative approach has been selective candidate gene studies that have focused upon single nucleotide polymorphisms (SNPs) within selected genes. It is well established that the activity of many genes may be modified by SNPs that normally occur with varied frequencies across populations. It is estimated that 10 million SNPs may regularly occur within the human genome and constitute 90% of the variation in a population. The most common patterns of these polymorphisms are currently being assessed in the International HapMap Project [74]. More than 30 SNPs have been associated with increased or decreased risks of preterm birth or preterm premature rupture of the membranes [75, 76]. Single nucleotide polymorphisms associated with preterm birth have predominately been in inflammatory and tissue remodeling pathways. Important ethnic differences in SNP frequencies may help explain racial disparities in preterm birth [77].
The role of genetic variation in stillbirth has not been explored.
Various environmental exposures have also been linked to poor pregnancy outcomes. The harmful effects of smoking during pregnancy are well established and it has been causally associated with preterm delivery and stillbirth (see article 3 on interventions).
Maternal serum and umbilical cord blood levels of pesticides such as dichlorodiphenyl trichloroethane (DDT) are associated in some, but not all, studies with preterm delivery [78]. Other organophosphate pesticide metabolites are associated with preterm birth at increasing exposure levels in the later part of pregnancy [79]. Finally, air pollution (particulate matter, carbon monoxide, lead, ozone, nitrogen dioxide, and sulfur dioxide) is associated with a variety of poor birth outcomes, including preterm birth [79]. Particulate matter is associated with small increases in preterm birth risk in studies from a number of countries such as China and the United States. Evidence is insufficient to infer causality between preterm birth and other air pollutants, but available data justifies further studies [80].
Investigation of interactions between genetics and environmental exposures are increasing, but is still limited. One recent example is the interaction between tobacco smoke exposure and preterm delivery [81, 82] Smoking is an important risk factor for preterm birth, but not all women exposed to the same level of smoke deliver preterm. Gene polymorphisms in cytochrome P-450 1A1 (CYP1A1) and glutathione S-transferase theta 1 (GSTT1) have been demonstrated to increase the risk of low birth weight and preterm birth among smokers, but not among non-smokers [81, 82]. These genes encode for enzymes involved in metabolism and detoxification of polycyclic aromatic hydrocarbon, an important carcinogen in tobacco smoke [82]. Women who smoke are also at increased risk of preterm delivery if they have certain factor V gene variants [83]. These women are hypothesized to be more prone to the procoagulant effects of tobacco smoke. Others have examined the interaction between air pollution and maternal genetics. Exposure to high levels of particulate matter during the third trimester in the presence of specific GSTT1 polymorphisms has been associated with a significantly increased risk of preterm delivery [84].
Similarly, gene polymorphisms for a variety of different inflammatory cytokines (e.g., tumor necrosis factor-a, interleukins-1, -6, -10, and their receptors and antagonists) have been associated with increased risks of preterm birth [76, 85] that may interact with environmental or infectious exposure. As noted above, Macones et al. noted an increased risk of preterm birth among women with bacterial vaginosis who also had a TNFα-308A allele polymorphism [32].
It is likely that many more examples of gene or environmental risk factors exist. Links between adverse pregnancy outcomes and a variety of environmental exposures have been discovered with a variable degree of strength. The data available are insufficient for many more potential exposure elements—due to limited exposure, limited human health effects, or both. There is a general lack of understanding of the dynamics of exposure during pregnancy; at what points in pregnancy is the mother/fetus most vulnerable, for example. A greater understanding is needed of the basic biological mechanisms of environmental influences on pregnancy and how they may interact with factors such as maternal genetics. Animal models may provide insight, but are limited by differences in critical periods of fetal development, genetics, and species-specific differences in suscept ibility to environmental exposures. Large-scale, well-designed and controlled human studies are necessary to establish the impact of environmental exposures on pregnancy outcomes.
Pathways to stillbirth
Stillbirth is a major contributor to perinatal mortality, with greater than 3 million stillbirths occurring annually worldwide (see article 1 [86]). Unfortunately, despite its global impact, research into etiologic mechanisms responsible for stillbirth has been hampered by the lack of standardized definitions and reporting [6]. At least 32 classification systems of stillbirth have been described [87]. Many of these have been developed for a specific purpose, and therefore differ in classifying causes, risk factors, and co-morbid conditions. Finally, the rate of "unexplained stillbirth" differs among settings depending upon available resources and interest. The etiology of stillbirths in low- and middle-income countries are frequently more difficult to ascertain. The cause of stillbirth is attributed by verbal autopsy and classified as macerated (antepartum) or fresh (intrapartum) [88] (see article 1 [86]). The rate of stillbirth is five-fold greater in low-income countries, where resources to evaluate stillbirths—such as trained birth attendants—are scarce.
It is likely that mechanisms responsible for preterm birth are also associated, in extremis, with stillbirth in many cases. In meticulous studies from Sweden, most stillbirths could be attributed to an etiology [89]. In that study, intrauterine infections, uteroplacental insufficiency, congenital malformations, placental abruption, and umbilical cord complications accounted for more than 50% of stillbirths (Figure 2). These are also important causes of preterm birth. Fewer than 20% of stillbirths could not be attributed to a specific etiology.
Representative causes of stillbirth in high-income countries. Source: Modifi ed from [90]. Reprinted from Acta Obstetricia et Gynecologica Scandinavica, 87, Varli H, Petersson K, Bottinga R, Bremme K, Hofsjo A, Holste C, Kublickas M, Norman M, Pilo C et al, The Stockholm classifi cation of stillbirth, 10, 2008, with permission from Acta Obstet Gynecol Scand.
In contrast, up to one-third of stillbirths in low- and middle-income countries are attributed to intrapartum asphyxia, and over 50% are "unexplained." The etiology of stillbirth also varies according to gestational age [90]. One study from Canada found that between 24 and 27 weeks of gestation the most common causes of stillbirth were infection (19%), placental abruption (14%), and fetal anomalies (14%). These are also important factors for preterm birth. Beyond 28 weeks of gestation, the most frequent causes of stillbirth in this study were related to placental abruption, fetal growth restriction, and maternal illnesses [91]. However, the contribution of these pathways to stillbirth in low- and middle-income countries is largely unknown. Clearly, further research in this very important area will depend upon a uniform classification and reporting system and better ascertainment in low- and middle-income countries.
New tools for reproductive research
One important limitation in understanding preterm births or stillbirths is the application of traditional biological methodologies to the complex process of parturition. Traditional biology attempts to explain complex phenomena by the functional properties of individual components of a complex system, a method described as "naive reductionism" [92]. Recent advances in highdimensional systems biology allow the characterization of complex processes through the global description of the components of a system and their interactions. New tools for systems biology research now exist:
-
computational design (or computer assisted in-silico modeling) [93]
-
genomics (characterization of genetic potential)
-
transcriptomics (characterization of gene expression)
-
proteomics (characterization of the protein output of gene transcription and translation)
-
metabolomics (characterization of the metabolic consequences of gene expression)
Collectively, these techniques have revolutionized biologic research in the last decade. A PubMed search reveals that over 39,000 articles have been published from 1998 through 2008 utilizing these techniques (search terms "functional genomics, transcriptome, proteomics, metabolomics). These techniques, however, have been only infrequently applied to pregnancy, with approximately
1,000 relevant articles published in the same time period (search term "+ pregnancy"). This disparity in utilization of these powerful research tools is increasing (Figure 3) and represents a critical gap in the understanding of parturition and stillbirths.
Nonetheless, gene expression studies of the pregnant uterus have contributed to the understanding of parturition [94–99] (Table 2). The assessment of gene expression is typically measured by detection of mRNA copies produced by each gene in what are known as microarray assays. These studies differed in design, gene expression platforms, and the number of genes studied. Yet collectively, these studies demonstrate that labor is a highly complex biological process, potentially involving hundreds of genes. The number of differentially expressed genes discovered, in fact, usually depends directly upon the number sought (Table 2). The most recent study, for example, using an Affymetrix platform to ascertain activity of over 12,000 genes found 110 genes that were up-regulated, and 29 that were down-regulated in association with labor [99]. Regardless of technique, microarray analysis has led to a new understanding of labor as inherently an inflammatory process, even in the absence of infection [97, 99]. Approximately 25-30% of genes—with up-regulated expression in labor—code for inflammatory proteins including chemokines, cytokines, extracellular remodeling proteins, and apoptosis. Thus, these studies have contributed to the preliminary understanding of events governing myometrial activation prior to labor.
In addition to functional genomics, it is well established that the activity of many genes may be modified by SNPs that normally occur with varied frequencies across populations. More than 30 SNPs have been associated with increased or decreased risk of preterm birth or preterm premature rupture of membranes [75]. Consistent with microarray data, the majority of the SNPs associated with preterm birth are in inflammatory, apoptotic, and tissue remodeling genes [75, 76] Thus, the application of systems biology, including functional genomics, transcriptomics, and SNP analysis, has contributed to a new paradigm: myometrial activation and the onset of myometrial stimulation is a highly complex genetically-controlled inflammatory process.
Proteomics has also made contributions in the understanding of abnormal parturition. Proteomics, a mass spectrometry-based technology, refers to a description of the protein complement of a system. Only 1-1.5% of the human genome codes for mRNA, and thus, leads to protein synthesis. These proteins, in turn, mediate cell-to-cell interactions in both health and disease. The major advantage of proteomics is that it most directly describes the functional output of a cell in both health and disease. Proteomics primarily aids biomarker discovery in the diagnosis of abnormal conditions of pregnancy that may contribute to prematurity. As noted by the March of Dimes, such diagnostic tools are urgently needed to facilitate timely intervention [100]. Proteomic analysis of amniotic fluid and cervical-vaginal secretions has been utilized to discover novel biomarkers for intra-amniotic infection [101, 102], spontaneous preterm birth [75, 103], and preterm premature rupture of the membranes [104]. Proteomic analysis of maternal urine has also identified several specific biomarkers for preeclampsia, an important cause of indicated preterm birth [75]. Additionally, comprehensive proteomic analysis has been utilized to fully characterize the protein complement of amniotic fluid and of cervical-vaginal fluid. Michaels, et al., utilizing LS/LS-MS/MS, identified 219 proteins in amniotic fluid, including 96 that were unique to amniotic fluid [105]. Similarly, Dasari, et al. identified a total of 150 unique proteins within cervical-vaginal fluid in pregnant women. Metabolism (32%) and immune response-related (22%) proteins were the major functional categories of proteins represented in the CVF proteome. A comparison of the CVF, serum, and amniotic fluid proteomes showed that 77 proteins are unique to CVF, while 56 and 17 CVF proteins also occur in serum and amniotic fluid, respectively [106]. The differential expression of proteins identified by these and other studies will likely provide the basis for diagnostic tests for many adverse pregnancy conditions including preterm birth.
Unfortunately, none of these news tools for systems biology has been applied to stillbirth. It is likely that application of these techniques in a systems biological approach will lead to significant contributions in the understanding of preterm birth and stillbirth. Because of the ability to study many processes simultaneously, however, systems biology depends upon carefully defined clinical phenotypes to avoid errors of misclassification and upon carefully prepared samples to avoid technical errors. Defining and correctly classifying differing phenotypes, or etiologies, of preterm birth or stillbirth—and appropriate collection and storage of biologic samples—are therefore critical needs and unmet gaps in systems biology and in understanding parturition.
Identification of critical gaps in discovery sciences
In addition to these general observations of gaps and research needs, several specific gaps are noted and outlined below. In contrast to epidemiological research where elegant matrixes have been proposed to prioritize research needs and the efficacy of interventions [107, 108], no such matrixes exist to assess potential benefits and risks of basic or translational science in reproductive biology. We therefore suggest an independent assessment tool to assess research gaps in basic and translational science to be applied prospectively in assessing basic and translational research needs (see Figure 4 and Additional File 1). This grading matrix, similar to CHNRI and GRADE is based upon the quality of evidence and the importance of outcomes (see Articles 1 and 3). Quality of evidence criteria include study design, soundness of methodology, consistency among or between studies, and biological rationale. A greater weight is given to human observations, consistency across species in animal models, and an established biologically plausible pathway. The importance of outcomes is based upon the ability to translate findings into clinically relevant trials, the strength of the effect, and the size of the population that may benefit. The final strength of the recommendation is therefore based upon the strength of the evidence of contribution to preterm birth or stillbirth, and the potential likelihood to yield significant benefits.
Specific gaps in basic science knowledge exist in all phases of parturition. Gaps that likely contribute to prematurity and stillbirth in which research is likely to yield significant benefit at each phase of parturition are summarized below.
Implantation
Perturbations in implantation have been associated with habitual abortions, stillbirths, and with preeclampsia, which is an important cause of medically-indicated pre-term birth [10]. There is a critical need for a better understanding of the immunologic regulation of trophoblast invasion, and how environmental factors including chronic intrauterine infection, or exposure to environmental toxicants (e.g., cigarette smoke and insecticides) or other xenobiotic agents that may influence implantation.
Quiescence
Progesterone is thought to be critical in maintaining uterine quiescence, and pharmacologic progesterone withdrawal is associated with increased uterine contractility. However, the influence of pollutants, xenobiotics, maternal intrauterine or systemic infections, or alterations in immune function upon progesterone (or other steroid hormone) synthesis and metabolism have not been well described, and would likely contribute to our understanding of preterm birth.
Activation and stimulation
Activation is a complex process characterized by increasing placental production of corticotrophin releasing hormone, activation of the fetal hypothalamic-pituitary-adrenal axis, progesterone withdrawal, and increasing estrogen and prostaglandin biosynthesis. This is a complex process—microarray studies of myometrium have demonstrated differential expression of more than 100 genes in the process of activation and stimulation [99]. Many factors can stimulate or usurp the normal mechanisms of activation, including stress, infection, hemorrhage, endocrine or immunologic abnormalities, and uterine overdistension. While the role and pathophysiology of infection-induced preterm labor or preterm labor associated with hemorrhage have been well-characterized, the pathophysiology of other initiating factors has not. Specifically, there are limited or no animal models for stress or distension in preterm birth, and this represents a critical research gap. In the setting of intrauterine infection, both the fetal genotype and the maternal genotype contribute to preterm birth. However, the role of fetal versus maternal contributions to preterm birth is largely unexplored in preterm birth that is not associated with infection. Finally, the role and pathophysiology of preterm birth and especially stillbirth in the setting of systemic maternal infection is not understood. This is especially relevant given that 50 million pregnant women suffer from malaria annually, and malaria represents an important cause of stillbirth and low birth weight in low- and middle-income countries.
Conclusion
Parturition is a continuous process beginning with implant ation and ending with involution of the uterus following birth. Active labor constitutes <0.5% of parturition. Preterm labor and stillbirth are common endpoints with multifactorial etiologies that perturb, usurp, or activate the normal processes of parturition. The physiologies of both normal and abnormal partur it ion remain poorly understood.
The following list includes critical gaps and needs in improving our understanding of these processes that are so important to survival:
-
Factors regulating implantation, and the potential impact of infection or xenobiotics in normal and abnormal placentation
-
Factors regulating uterine quiescence, and the role of progesterone in preventing preterm birth
-
Mechanisms responsible for activation of the myometrium from a state of quiescence to a state of contractility
-
Appropriate animal models to replicate pathway-specific etiologies of preterm birth or stillbirth that will contribute to rational and efficacious interventions
-
The role of environmental exposure and xenobiotics in activation and stimulation of contractions
-
The contributions of both the fetal and the maternal genotype in determining the clinical outcome (i.e., the phenotype) of preterm labor and stillbirth
-
The role of gene:environment interactions in preterm birth and stillbirth
-
The pathophysiology of common systemic infections like malaria in preterm birth and stillbirth
-
The application of systems biology to better understand the complexity of parturition
In all of these critical needs, it is important to recognize that preterm birth and stillbirth are complex syndromes with many etiologies and pathways. In the past, research has been hampered by failure to recognize this heterogeneity and has led to conflicting findings. The greatest immediate needs to facilitate advances in the above gaps in knowledge include the following: carefully defined phenotypes associated with preterm birth and stillbirth; standardized definitions; uniform criteria for assessing outcomes; and the collection of biological specimens.
The next articles in this report address existing interventions [109], scale-up [110], advocacy [111], and ethics [112]. The final article presents a Global Action Agenda developed by nearly 200 global stakeholders [113]. It includes specific objectives related to normal and abnormal gestational biology, genetics, and the environment. Each is set to a specific short-, intermediate, or long-term timeline.
References
Romero R, Kuivaniemi H, Tromp G: Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab. 2002, 87 (6): 2431-2434. 10.1210/jc.87.6.2431.
Challis JRG: Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000, 55 (10): 650-660. 10.1097/00006254-200010000-00025.
Challis JRG, Matthews SG, Gibb W, Lye SJ: Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000, 21 (5): 514-550. 10.1210/er.21.5.514.
Behrman RE, Butler AS: Institute of Medicine, Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. 2007, The National Academies Press
Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M: The preterm parturition syndrome. BJOG. 2006, 113 (Suppl 3): 17-42.
Smith GC, Fretts RC: Stillbirth. Lancet. 2007, 370 (9600): 1715-1725. 10.1016/S0140-6736(07)61723-1.
Goldenberg RL, Culhane JF, lams JD, Romero R: Epidemiology and causes of preterm birth. Lancet. 2008, 371 (9606): 75-84. 10.1016/S0140-6736(08)60074-4.
Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM: Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol. 2005, 105: 1084-1091.
MacDonald PC, Casey ML: Preterm birth. Sci Am Sci Med. 1996, 3: 42-51.
Eastabrook G, Hu Y, von Dadelszen P: The role of decidual natural killer cells in normal placentation and in the pathogenesis of preeclampsia. J Obstet Gynaecol Can. 2008, 30 (6): 467-476.
Garfield R, C. J. McNellis D, MacDonald PC, Nathanielsz PW, Roberts JM: Structural and functional studies of the control of myometrial contractility and labour. The Onset of Labour: Cellular and Integrative Mechansims. Perinatology Press. 1988, 55-80.
Lye SJ, Ou CW, Teoh TG, et al: The molecular basis of labour and tocolysis. Fetal Maternal Med Rev:. 1998, 121-136. 10.1017/S096553959800031X.
Smith R, Mesiano S, McGrath S: Hormone trajectories leading to human birth. Regul Pept. 2002, 108 (2-3): 159-164. 10.1016/S0167-0115(02)00105-2.
McLean M, Smith R: Corticotropin-releasing Hormone in Human Pregnancy and Parturition. Trends Endocrinol Metab. 1999, 10 (5): 174-178. 10.1016/S1043-2760(98)00146-5.
Word RA, Li XH, Hnat M, Carrick K: Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007, 25: 69-79. 10.1055/s-2006-956777.
Villar J, Abalos E, Carroli G, Giordano D, Wojdyla D, Piaggio G, Campodonico L, Gulmezoglu M, Lumbiganon P, Bergsjo P, et al: Heterogeneity of perinatal outcomes in the preterm delivery syndrome. Obstet Gynecol. 2004, 104 (1): 78-87.
Barros FC, Velez Mdel P: Temporal trends of preterm birth subtypes and neonatal outcomes. Obstet Gynecol. 2006, 107 (5): 1035-1041.
Lockwood CJ, Kuczynski E: Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001, 15 (Suppl 2): 78-89. 10.1046/j.1365-3016.2001.00010.x.
Goldenberg RL, Hauth JC, Andrews WW: Intrauterine infection and preterm delivery. N Engl J Med. 2000, 342 (20): 1500-1507. 10.1056/NEJM200005183422007.
Hauth JC, Andrews WW, Goldenberg RL: Infection-related risk factors predictive of spontaneous preterm birth. Prenat Neonat Med. 1998, 3: 86-90.
Watts DH, Krohn MA, Hillier SL, Eschenbach DA: The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992, 79 (3): 351-357. 10.1097/00006250-199203000-00005.
Romero R, Avila C, Brekus CA, Morotti R: The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991, 622: 355-375. 10.1111/j.1749-6632.1991.tb37880.x.
Romero R, Erez O, Espinoza J: Intrauterine infection, preterm labor, and cytokines. J Soc Gynecol Investig. 2005, 12 (7): 463-465. 10.1016/j.jsgi.2005.09.001.
Kimberlin DF, Andrews WW: Bacterial vaginosis: association with adverse pregnancy outcome. Semin Perinatol. 1998, 22 (4): 242-250. 10.1016/S0146-0005(98)80012-8.
Donati L, Di Vico A, Nucci M, Quagliozzi L, Spagnuolo T, Labianca A, Bracaglia M, lanniello F, Caruso A, Paradisi G: Vaginal microbial flora and outcome of pregnancy. Arch Gynecol Obstet. 2009
McDonald HM, Brocklehurst P, Gordon A: Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007, CD000262-1
Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J: Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996, 67 (10 Suppl): 1103-1113.
Xiong X, Buekens P, Vastardis S, Yu SM: Periodontal disease and pregnancy outcomes: state-of-the-science. Obstet Gynecol Surv. 2007, 62 (9): 605-615. 10.1097/01.ogx.0000279292.63435.40.
Shub A, Swain JR, Newnham JP: Periodontal disease and adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2006, 19 (9): 521-528. 10.1080/14767050600797749.
Socransky SS, Haffajee AD: Periodontal microbial ecology. Periodontol 2000. 2005, 38: 135-187. 10.1111/j.1600-0757.2005.00107.x.
Fredricks DN, Fiedler TL, Marrazzo JM: Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005, 353 (18): 1899-1911. 10.1056/NEJMoa043802.
Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF: A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004, 190 (6): 1504-1508. 10.1016/j.ajog.2004.01.001. discussion 1503A
Sullivan AD, Nyirenda T, Cullinan T, Taylor T, Harlow SD, James SA, Meshnick SR: Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. J Infect Dis. 1999, 179 (6): 1580-1583. 10.1086/314752.
Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, Bulmer J, Balira R, Ross D, Mugeye K, Hayes R, et al: Adverse birth outcomes in United Republic of Tanzania-impact and prevention of maternal risk factors. Bull World Health Organ. 2007, 85 (1): 9-18.
Newman RD, Hailemariam A, Jimma D, Degifie A, Kebede D, Rietveld AE, Nahlen BL, Barnwell JW, Steketee RW, Parise ME: Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a nonepidemic year. J Infect Dis. 2003, 187 (1 1): 1765-1772. 10.1086/374878.
Osman NB, Challis K, Cotiro M, Nordahl G, Bergstrom S: Perinatal outcome in an obstetric cohort of Mozambican women. J Trop Pediatr. 2001, 47 (1): 30-38. 10.1093/tropej/47.1.30.
Luxemburger C, McGready R, Kham A, Morison L, Cho T, Chongsuphajaisiddhi T, White NJ, Nosten F: Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am J Epidemiol. 2001, 154 (5): 459-465. 10.1093/aje/154.5.459.
Allen S, Raiko A, O’Donnell A, et al: Causes of preterm delivery and intrauterine growth retardation in a malaria endemic region of Papua new Guinea. Archives of Diseases in Childhood. 1998, 79: 135-140.
Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D’Alessandro U, Nosten F, et al: The sick placenta-the role of malaria. Placenta. 2004, 25 (5): 359-378. 10.1016/j.placenta.2003.10.019.
Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW: Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007, 7 (2): 105-117. 10.1016/S1473-3099(07)70022-1.
Finelli L, Berman SM, Koumans EH, Levine WC: Congenital syphilis. Bull World Health Organ. 1998, 76 (Suppl 2): 126-128.
Berman SM: Maternal syphilis: pathophysiology and treatment. Bull World Health Organ. 2004, 82 (6): 433-438.
Peeling RW, Hook EW: The pathogenesis of syphilis: the Great Mimicker, revisited. J Pathol. 2006, 208 (2): 224-232. 10.1002/path.1903.
Wicher V, Wicher K: Pathogenesis of maternal-fetal syphilis revisited. Clin Infect Dis. 2001, 33 (3): 354-363. 10.1086/321904.
Arias F, Rodriguez L, Rayne SC, Krauss FT: Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993, 168 (2): 585-591.
Elovitz MA, Ascher-Landsberg J, Saunders T, Phillippe M: The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am J Obstet Gynecol. 2000, 183 (3): 674-681. 10.1067/mob.2000.106751.
Mackenzie AP, Schatz F, Krikun G, Funai EF, Kadner S, Lockwood CJ: Mechanisms of abruption-induced premature rupture of the fetal membranes: Thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am J Obstet Gynecol. 2004, 191 (6): 1996-2001. 10.1016/j.ajog.2004.08.003.
Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ: Thrombin- enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med. 2002, 11 (1): 11-17.
Stephenson CD, Lockwood CJ, Ma Y, Guller S: Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J Matern Fetal Neonatal Med. 2005, 18 (1): 17-22. 10.1080/14767050500123632.
Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F: Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin-enhanced interleukin-8 expression in term decidua. Am J Pathol. 2005, 167 (5): 1443-1449.
Austin MP, Leader L: Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol. 2000, 40 (3): 331-337. 10.1111/j.1479-828X.2000.tb03344.x.
Wadhwa PD, Culhane JF, Rauh V, Barve SS: Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001, 5 (2): 119-125. 10.1023/A:1011353216619.
Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP: Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks' gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999, 180 (1 Pt 3): S257-263. 10.1016/S0002-9378(99)70712-X.
Hobel CJ, Dunkel-Schetter C, Roesch S: Maternal stress as a signal to the fetus. Prenatal and Neonatal Medicine. 1998, 3: 116-120.
Ou CW, Orsino A, Lye SJ: Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997, 138 (12): 5398-5407. 10.1210/en.138.12.5398.
Terzidou V, Sooranna SR, Kim LU, Thornton S, Bennett PR, Johnson MR: Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J Clin Endocrinol Metab. 2005, 90 (1): 237-246. 10.1210/jc.2004-0277.
Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR: Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod. 2004, 10 (2): 109-113. 10.1093/molehr/gah021.
Maradny EE, Kanayama N, Halim A, Maehara K, Terao T: Stretching of fetal membranes increases the concentration of interleukin-8 and collagenase activity. Am J Obstet Gynecol. 1996, 174 (3): 843-849. 10.1016/S0002-9378(96)70311-3.
Loudon JA, Sooranna SR, Bennett PR, Johnson MR: Mechanical stretch of human uterine smooth muscle cells increases IL-8 mRNA expression and peptide synthesis. Mol Hum Reprod. 2004, 10 (12): 895-899. 10.1093/molehr/gah112.
Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P: Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995, 172 (4Pt1): 1097-1103. 10.1016/0002-9378(95)91469-2. discussion 1104-1096
Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, et al: The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996, 334 (9): 567-572. 10.1056/NEJM199602293340904.
Romero R, Espinoza J, Erez O, Hassan S: The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified?. Am J Obstet Gynecol. 2006, 194 (1): 1-9. 10.1016/j.ajog.2005.12.002.
Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM: Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000, 182 (6): 1458-1467. 10.1067/mob.2000.106851.
Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB: Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992, 167 (4 Pt 1): 1086-1091.
Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, et al: A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006, 34 (1): 13-19. 10.1515/JPM.2006.002.
Shubert PJ, Diss E, Iams JD: Etiology of preterm premature rupture of membranes. Obstet Gynecol Clin North Am. 1992, 19 (2): 251-263.
Menon R, Fortunato SJ: The role of matrix degrading enzymes and apoptosis in rupture of membranes. JSoc Gynecol Investig. 2004, 11 (7): 427-437. 10.1016/j.jsgi.2004.04.001.
Fortunato SJ, Menon R, Bryant C, Lombardi SJ: Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet Gynecol. 2000, 182 (6): 1468-1476. 10.1067/mob.2000.107330.
Vadillo-Ortega F, Sadowsky DW, Haluska GJ, Hernandez-Guerrero C, Guevara-Silva R, Gravett MG, Novy MJ: Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002, 186 (1): 128-138. 10.1067/mob.2002.118916.
So T: [The role of matrix metalloproteinases for premature rupture of the membranes]. Nippon Sanka Fujinka Gakkai Zasshi. 1993, 45 (3): 227-233.
Menon R: Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet GynecolScand. 2008, 87 (6): 590-600. 10.1080/00016340802005126.
Pennell CE, Jacobsson B, Williams SM, Buus RM, Muglia LJ, Dolan SM, Morken NH, Ozcelik H, Lye SJ, Relton C: Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007, 196 (2): 107-118. 10.1016/j.ajog.2006.03.109.
Clausson B, Lichtenstein P, Cnattingius S: Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000, 107 (3): 375-381. 10.1111/j.1471-0528.2000.tb13234.x.
Consortium TIH: The international HapMap project. Nature. 2003, 426: 789-796. 10.1038/nature02168.
Buhimschi CS, Rosenberg VA, Dulay AT, Thung S, Sfakianaki AK, Bahtiyar MO, Buhimschi IA: Multidimensional system biology: genetic markers and proteomic biomarkers of adverse pregnancy outcome in preterm birth. Am J Perinatol. 2008, 25 (3): 175-187. 10.1055/s-2008-1061497.
Crider KS, Whitehead N, Buus RM: Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005, 7 (9): 593-604. 10.1097/01.gim.0000187223.69947.db.
Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, Thorsen P: If Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reprod Biol Endocrinol. 2009, 7: 62-10.1186/1477-7827-7-62.
Windham G, Fenster L: Environmental contaminants and pregnancy outcomes. Fertil Steril. 2008, 89 (2Suppl): e111-116. 10.1016/j.fertnstert.2007.12.041. discussion e117
Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ: Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008, 15 (7): 631-650. 10.1177/1933719108322436.
Stram RJ, Binkova B, Dejmek J, Bobak M: Ambient air pollution and prenancy outcomes: a review of the literature. Environ Health Perspect. 2005, 133 (4): 375-382.
Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X: Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002, 287 (2): 195-202. 10.1001/jama.287.2.195.
Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, Pearson C, Ortiz K, Zuckerman B, Bauchner H, et al: Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on GxE interactions and pathogenic pathways. Hum Genet. 2008, 123 (4): 359-369. 10.1007/s00439-008-0485-9.
Yu Y, Tsai HJ, Liu X, Mestan K, Zhang S, Pearson C, Ortiz K, Xu X, Zuckerman B, Wang X: The joint association between F5 gene polymorphisms and maternal smoking during pregnancy on preterm delivery. Hum Genet. 2009, 124 (6): 659-668. 10.1007/s00439-008-0589-2.
Suh YJ, Ha EH, Park H, Kim YJ, Kim H, Hong YC: GSTM1 polymorphism along with PM10 exposure contributes to the risk of preterm delivery. Mutat Res. 2008, 656 (1-2): 62-67.
Anum EA, Springel EH, Shriver MD, Strauss JF: Genetic contributions to disparities in preterm birth. Pediatr Res. 2009, 65: 1-9. 10.1203/PDR.0b013e31818912e7.
Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, and GAPPS Review Group: Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S1-
Reddy UM, Goldenberg R, Silver R, Smith GC, Pauli RM, Wapner RJ, Gardosi J, Pinar H, Grafe M, Kupferminc M, et al: Stillbirth classification-developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009, 114 (4): 901-914. 10.1097/AOG.0b013e3181b8f6e4.
Lawn JE, Yakoob MY, Haws RA, Soomro T, Darmstadt GL, Bhutta ZA: 3.2 million stillbirths: epidemiology and overview of the evidence review. BMC Pregnancy Childbirth. 2009, 9 (Suppl 1): S2-10.1186/1471-2393-9-S1-S2.
Petersson K, Bremme K, Bottinga R, Hofsjo A, Hulthen-Varli I, Kublickas M, Norman M, Papadogiannakis N, Wanggren K, Wolff K: Diagnostic evaluation of intrauterine fetal deaths in Stockholm 1998-99. Acta Obstet Gynecol Scand. 2002, 81 (4): 284-292.
Varli IH, Petersson K, Bottinga R, Bremme K, Hofsjo A, Holm M, Holste C, Kublickas M, Norman M, Pilo C, et al: The Stockholm classification of stillbirth. Acta Obstet Gynecol Scand. 2008, 87 (11): 1202-1212. 10.1080/00016340802460271.
Fretts RC: Etiology and prevention of stillbirth. A m J Obstet Gynecol. 2005, 193 (6): 1923-1935. 10.1016/j.ajog.2005.03.074.
Bloom F: What does it all mean to you?. J Neurosci. 2001, 21 (21): 8304-8305.
Klauschen F, Angermann BR, Meier-Schellersheim M: Understanding diseases by mouse click: the promise and potential of computational approaches in Systems Biology. Clin Exp Immunol. 2007, 149 (3): 424-429.
Aguan KCJ, Thompson LP, et al: Applications of a functional genomics approach to identify differentially expressed genes in human myometrium during pregnancy and labour. Mol Hum Reprod. 2000, 6: 1141-1145. 10.1093/molehr/6.12.1141.
Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, Smith R: Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab. 2002, 87 (6): 2435-2441. 10.1210/jc.87.6.2435.
Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, Adashi EY, Whiting D: The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol. 2005, 193 (2): 404-413. 10.1016/j.ajog.2004.12.021.
Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA: Human myometrial gene expression before and during parturition. Biol Reprod. 2005, 72 (3): 707-719. 10.1095/biolreprod.104.032979.
Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S: Labor- associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006, 3 (6): e169-10.1371/journal.pmed.0030169.
Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S: Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009, 200 (1): 104 e101-111.
Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, Merkatz IR, Greene MF, Schwarz RH: Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005, 193 (3 Pt 1): 626-635. 10.1016/j.ajog.2005.02.106.
Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, et al: Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004, 292 (4): 462-469. 10.1001/jama.292.4.462.
Gravett MG, Thomas A, Schneider KA, Reddy AP, Dasari S, Jacob T, Lu X, Rodland M, Pereira L, Sadowsky DW, et al: Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. 2007, 6 (1): 89-96. 10.1021/pr060149v.
Pereira L, Reddy AP, Jacob T, Thomas A, Schneider KA, Dasari S, Lapidus JA, Lu X, Rodland M, Roberts CT, et al: Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007, 6 (4): 1269-1276. 10.1021/pr0605421.
Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, Bienvenut WV, Lemery D, Quadroni M, Dastugue B, et al: Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003, 3 (8): 1521-1525. 10.1002/pmic.200300455.
Michaels JE, Dasari S, Pereira L, Reddy AP, Lapidus JA, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, et al: Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational age-dependent changes. J Proteome Res. 2007, 6 (4): 1277-1285. 10.1021/pr060543t.
Dasari S, Pereira L, Reddy AP, Michaels JE, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Gravett MG, et al: Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome. Res. 2007, 6 (4): 1258-1268. 10.1021/pr0605419.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, et al: Grading quality of evidence and strength of recommendations. BMJ. 2004, 328 (7454): 1490-10.1136/bmj.328.7454.1490.
Rudan I, Gibson JL, Ameratunga S, El Arifeen S, Bhutta ZA, Black M, Black RE, Brown KH, Campbell H, Carneiro I, et al: Setting priorities in global child health research investments: guidelines for implementation of CHNRI method. Croat Med J. 2008, 49 (6): 720-733. 10.3325/cmj.2008.49.720.
Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE, and GAPPS Review Group: Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S3-
Victora CG, Rubens CE, and the GAPPS Review Group: Global report on preterm birth and stillbirth (4 of 7): delivery of interventions. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S4-
Sather M, Fajon AV, Zaentz R, Rubens CE, and the GAPPS Review Group: Global report on preterm birth and stillbirth (5 of 7): advocacy barriers and opportunities. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S5-
Kelley MK, Rubens CE, and the GAPPS Review Group: Global report on preterm birth and stillbirth (6 of 7): ethical considerations. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S6-
Rubens CE, Gravett MG, Victora CG, Nunes TM, and the GAPPS Review Group: Global report on preterm birth and stillbirth (7 of 7): mobilizing resources to accelerate innovative interventions. BMC Pregnancy and Childbirth. 2010, 10 (Suppl 1): S7-
Acknowledgements
This report was supported by the Global Alliance to Prevent Prematurity and Stillbirth, an initiative of Seattle Children's, through a grant from the Bill & Melinda Gates Foundation. We also thank Catherine Waszak for her outstanding administrative support.
This article has been published as part of BMC Pregnancy and Childbirth Volume 10 Supplement 1, 2010. The full contents of this report are available online at http://www.biomedcentral.com/1471-2393/10?issue=S1. We thank all members of the GAPPS Review Group for their contributions and review of the seven articles in this report, and list them here in alphabetical order: Fernando C Barros, Maneesh Batra, Zulfiqar Ahmed Bhutta, Anne-Véronique Fajon, Michael G Gravett, Thomas N Hansen, Maureen Kelley, Joy E Lawn, Toni M Nunes, Craig E Rubens, Megan Sather, Cynthia Stanton, Cesar G Victora, and Rachel Zaentz.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Authors' contributions
The article was written by MGG. CER helped conceive of this article as part of a global report on preterm birth and stillbirth, and participated in its design, coordination, and review. TMN also helped with the coordination and review, and edited the article.
Competing interests
The authors declare they have no competing interests.
Electronic supplementary material
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gravett, M.G., Rubens, C.E., Nunes, T.M. et al. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth 10 (Suppl 1), S2 (2010). https://doi.org/10.1186/1471-2393-10-S1-S2
Published:
DOI: https://doi.org/10.1186/1471-2393-10-S1-S2