Abstract
Background
Patients with multiple sclerosis (MS) have a decreased frequency of CD8+ T cells reactive to their own Epstein-Barr virus (EBV) infected B cells. We have proposed that this might predispose to the development of MS by allowing EBV-infected autoreactive B cells to accumulate in the central nervous system. The decreased CD8+ T cell response to EBV results from a general CD8+ T cell deficiency and also a decreased proportion of EBV-specific T cells within the total CD8+ T cell population. Because decreased HLA class I expression on monocytes and B cells has been reported in MS and could influence the generation and effector function of EBV-specific CD8+ T cells, the present study was undertaken to measure the expression of HLA molecules on B cells and monocytes in patients with MS.
Methods
We used flow cytometry to determine the proportions of T cells, natural killer cells, B cells and monocytes in peripheral blood mononuclear cells (PBMC) and to quantify the expression of HLA molecules on T cells, B cells and monocytes of 59 healthy subjects and 62 patients with MS who had not received corticosteroids or immunomodulatory therapy in the previous 3 months.
Results
The levels of HLA class I and class II molecules expressed on T cells, B cells and monocytes were normal in patients with MS, with the exception of two patients with secondary progressive MS with very low class II expression on B cells. In confirmation of previous studies we also found that the percentage of CD8+ T cells was significantly decreased whereas the percentage of CD4+ T cells and the CD4:CD8 ratio were significantly increased in patients with MS compared to healthy subjects.
Conclusions
The decreased CD8+ T cell response to EBV-infected B cells in MS patients is not due to decreased HLA class I expression on monocytes or B cells. In a small proportion of patients decreased HLA class II expression on B cells might impair the CD8+ T cell response to EBV by reducing CD4+ T cell help.
Similar content being viewed by others
Background
A large body of evidence indicates that infection with the Epstein-Barr virus (EBV) has a role in the pathogenesis of multiple sclerosis (MS) [1–3]. Several hypotheses have been proposed to explain the role of EBV in the development of MS, including immunological crossreactivity between EBV and central nervous system (CNS) antigens [4], autoimmunity to αB-crystallin [5], EBV infection of autoreactive B cells [6], bystander damage from immune attack against EBV in the CNS [7], and interactions with other infectious agents such as human endogenous retroviruses [8]. It is important to determine the role of EBV in the pathogenesis of MS because of the potential to prevent and treat MS by controlling EBV infection, such as by vaccination against EBV [9]. In particular it is necessary to understand the exact relationship between EBV and the immune system in MS in order to ensure that these interventions are beneficial, not harmful.
EBV infection is normally kept under tight control by EBV-specific immune responses, especially by cytotoxic CD8+ T cells which eliminate proliferating and lytically infected B cells [10]. We have hypothesized that a genetically determined defect in the elimination of EBV-infected B cells by cytotoxic CD8+ T cells might predispose to the development of MS by allowing EBV-infected autoreactive B cells to accumulate in the central nervous system [3, 6]. Studies of CD8+ T cell reactivity to EBV using selected synthetic EBV peptides to stimulate the T cells have produced conflicting results, with reports of normal reactivity [11] and increased reactivity [12] in clinically isolated syndromes, and normal reactivity [12, 13] and increased reactivity [14] in established MS. Studies using selected EBV peptides to assess T cell reactivity are limited by the fact that they do not provide a measure of the total T cell response to EBV, which encodes many different proteins. To overcome this it is necessary to measure the T cell response to autologous EBV-infected B cells. This provides a direct measure of the aggregate T cell response to EBV-infected B cells in each subject because it uses each person's natural antigen-processing mechanisms to present viral antigens at normal physiological concentrations on the surface of their own EBV-infected B cells and it represents the total T cell response to all EBV antigens presented by all HLA molecules on infected B cells in each subject [15]. Using this approach we have shown that patients with MS have a decreased frequency of CD8+ T cells reactive to their own EBV-infected B cell lymphoblastoid cell lines (LCL) [15]. This results from a general CD8+ T cell deficiency and also a decreased proportion of EBV-specific T cells within the total CD8+ T cell population [16]. Our finding of decreased CD8+ T cell reactivity to LCL in patients with MS [15] differs from a previous small study on 11 patients which reported a non-significant increase in the frequency of LCL-specific CD8+ T cells [17], but is consistent with an early report of decreased T cell control of LCL outgrowth [18] and a recent report of a trend (p = 0.07) towards a decreased CD8+ T cell response to LCL [19].
In view of a previous report of decreased HLA class I expression on monocytes and B cells in patients with MS [20], which could impair the generation and effector function of EBV-specific CD8+ T cells, we have undertaken the present study to quantify the level of HLA expression on B cells and monocytes in MS patients and healthy subjects.
Methods
Patients and controls
Blood was collected from 59 healthy subjects and 62 MS patients following informed consent. This study was approved by the Royal Brisbane & Women's Hospital Human Research Ethics Committee and The University of Queensland Medical Research Ethics Committee. All patients met the 2005 Revised McDonald Criteria for a diagnosis of MS [21]. The clinical course was relapsing-remitting (RRMS) in 23 patients, secondary progressive (SPMS) in 25 and primary progressive (PPMS) in 14. The patients had not received corticosteroids or immunomodulatory therapy for at least 3 months prior to venesection. Only two patients had ever received immunosuppressive drugs and these had been ceased four years before blood collection. An additional 7 patients had received interferon-β which had been ceased 6 months to 6 years before blood collection. Disability was assessed using the Kurtzke Expanded Disability Status Scale (EDSS) [22], and the MS Severity Score (MSSS) was determined from the EDSS and disease duration [23]. The demographic and clinical details of the healthy subjects and patients with MS are presented in Table 1.
Flow cytometry
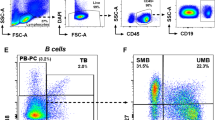
Peripheral blood mononuclear cells (PBMC) were separated by density centrifugation and cryopreserved, as previously described [15]. PBMC samples were thawed and cultured for 24 h to allow cells to rest and re-express cell surface receptors. Propidium iodide staining demonstrated > 99% viability within the lymphocyte and monocyte forward scatter/side scatter gate for each sample used. PBMC samples were assessed using a Becton Dickinson FACSCalibur flow cytometer to determine the percentages of CD3+ T cells, CD4+CD3+ T cells, CD8+CD3+ T cells, CD16+CD3-/CD56+CD3- natural killer (NK) cells, CD19+ B cells and CD14+ monocytes within the combined lymphocyte and monocyte gates. To quantify expression of HLA class I or class II molecules, PBMC were co-stained with either anti-HLA-ABC or anti-HLA-DR/DP/DQ in combination with anti-CD3 (T cells), anti-CD19 (B cells) or anti-CD14 (monocyte) antibodies. HLA-DR expression was also measured in 30 of the healthy subjects and 33 of the MS patients. Calibrite beads (BD Pharmingen) and FACSComp software were used daily to ensure calibrated, comparable measurement of geometric mean fluorescence intensity (MFI). Antibodies were directly conjugated to allophycocyanin (anti-CD3, anti-CD19 and anti-CD14), R-phycoerythrin (anti-CD4, anti-CD8, anti-CD16 and anti-CD56) or fluorescein isothiocyanate (anti-HLA-ABC, anti-HLA-DR/DP/DQ and anti-HLA-DR) (BD Pharmingen, San Diego, California, USA). CellQuest software (BD Biosciences) was used for acquisition and analysis of flow cytometry data.
Data analysis and statistics
Statistical analyses were performed using GraphPad Prism version 5.04 (Graphpad Software Inc., San Diego, California, USA, http://www.graphpad.com). For comparisons between the whole group of MS patients and healthy subjects, Student's t test or the Mann-Whitney rank sum test was used, according to the distribution of the data. For comparison between each of the subtypes of MS (RRMS, SPMS and PPMS) and healthy subjects, one-way analysis of variance with Dunnett's test for multiple comparisons was used. The CD4:CD8 ratios were log transformed to make the distributions approximately normal prior to analysis. Differences were considered significant for p < 0.05.
Results
Table 2 shows the proportions of lymphocytes and monocytes in the peripheral blood. The percentages of CD3+ T cells, NK cells, B cells and monocytes were normal in the total group of MS patients, as well as in the RRMS, SPMS and PPMS subgroups, with the exception of an increased percentage of CD3+ T cells in PPMS. The percentage of CD8+ T cells was significantly decreased whereas the percentage of CD4+ T cells and the CD4:CD8 ratio were significantly increased in the total group of MS patients compared to healthy subjects; these changes were more pronounced in SPMS and PPMS than in RRMS. The levels of expression of HLA class I molecules on T cells, B cells and monocytes were normal in the total group of MS patients and in the RRMS, SPMS and PPMS subgroups (Table 2). Analysis of HLA class I expression with the W6/32 antibody used by Li and colleagues [20] also demonstrated normal HLA class I expression on lymphocytes and monocytes in the MS patients (data not shown). The levels of HLA class II expression on T cells, B cells and monocytes were also normal in the total group of MS patients, as well as in the RRMS, SPMS and PPMS subgroups (Table 2), with the exception of very low levels of B cell expression (MFI = 1077 and 1139) in 2 patients with SPMS. HLA-DR expression on B cells was also assessed in one of these 2 patients and found to be very low (MFI = 2404). HLA-DR expression on B cells and monocytes was otherwise normal in the MS patients tested (Table 3).
Discussion
In the present study we have found that patients with MS have normal expression of HLA class I molecules on T cells, B cells and monocytes. Our findings stand in contrast to a previous study reporting low levels of HLA class I expression on T cells, B cells and monocytes in MS patients [20], but are consistent with a report of normal HLA class I expression on monocytes in MS [24] and with our previous finding of normal HLA class I expression on EBV-infected LCL in MS patients [15]. We therefore conclude that the decreased CD8+ T cell response to EBV-infected B cells in MS patients [15, 16] is not due to decreased HLA class I expression on monocytes or B cells.
We also found normal expression of HLA class II molecules on T cells, B cells and monocytes in patients with MS, with the exception of two patients with SPMS who had very low class II expression on B cells. Low B cell HLA class II expression might impair the CD8+ T cell response to EBV by reducing CD4+ T cell help, which normally stimulates the expansion of EBV-specific CD8+ T cells by producing interleukin-2 [25]. Our finding of normal HLA-DR expression on monocytes in MS is consistent with the report of Kouwenhoven and colleagues [24].
Our findings of an increased proportion of CD4+ T cells, a decreased proportion of CD8+ T cells and an increased CD4:CD8 ratio in the blood of patients with MS are consistent with previous studies [18, 26–33]. Our observation that CD8+ T cell deficiency is more marked in SPMS and PPMS than in RRMS corroborates previous studies reporting that the decrease in CD8+ T cells is most pronounced in progressive MS [29–31]. We did not measure the absolute counts of CD4+ T cells and CD8+ T cells but previous studies have shown that the absolute number of CD4+ T cells is normal and the absolute number of CD8+ T cells is decreased in MS [30, 32, 33]. Future studies should be directed towards determining the cause of the CD8+ T cell deficiency, which we propose is genetically determined. The CD4/CD8 T cell ratio is genetically controlled [34], with at least some of the responsible genes being located in the HLA complex [35]. CD8+ T cell deficiency is a feature of many chronic autoimmune diseases and is also found in healthy blood relatives of patients with autoimmune diseases [36–38], indicating that it is genetically determined and not secondary to the disease process.
Conclusions
The decreased CD8+ T cell response to EBV-infected B cells in MS patients is not due to decreased HLA class I expression on monocytes or B cells. In a small proportion of patients decreased HLA class II expression on B cells might impair the CD8+ T cell response to EBV by reducing CD4+ T cell help.
References
Ascherio A, Munger KL: Epstein-Barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol. 2010, 5: 271-277. 10.1007/s11481-010-9201-3.
Santiago O, Gutierrez J, Sorlozano A, de Dios Luna J, Villegas E, Fernandez O: Relation between Epstein-Barr virus and multiple sclerosis: analytic study of scientific production. Eur J Clin Microbiol Infect Dis. 2010, 29: 857-866. 10.1007/s10096-010-0940-0.
Pender MP: The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist. 2011, 17: 351-367. 10.1177/1073858410381531.
Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, Stuart DI, Bell JI, Jones EY, Fugger L: A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002, 3: 940-943. 10.1038/ni835.
Van Noort JM, Bajramovic JJ, Plomp AC, van Stipdonk MJB: Mistaken self, a novel model that links microbial infections with myelin-directed autoimmunity in multiple sclerosis. J Neuroimmunol. 2000, 105: 46-57. 10.1016/S0165-5728(00)00181-8.
Pender MP: Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003, 24: 584-588. 10.1016/j.it.2003.09.005.
Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, Andreoni L, Trivedi P, Salvetti M, Faggioni A, Aloisi F: Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007, 204: 2899-2912. 10.1084/jem.20071030.
Krone B, Grange JM: Multiple sclerosis: are protective immune mechanisms compromised by a complex infectious background?. Autoimmune Dis. 2010, 2011: 708750-
Pender MP: Preventing and curing multiple sclerosis by controlling Epstein-Barr virus infection. Autoimmun Rev. 2009, 8: 563-568. 10.1016/j.autrev.2009.01.017.
Hislop AD, Taylor GS, Sauce D, Rickinson AB: Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007, 25: 587-617. 10.1146/annurev.immunol.25.022106.141553.
Lünemann JD, Tintoré M, Messmer B, Strowig T, Rovira A, Perkal H, Caballero E, Münz C, Montalban X, Comabella M: Elevated Epstein-Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol. 2010, 67: 159-169. 10.1002/ana.21886.
Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A, Kleeberg J, Le Goff G, Pantaleo G, Du Pasquier RA: Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008, 131: 1712-1721. 10.1093/brain/awn108.
Gronen F, Ruprecht K, Weissbrich B, Klinker E, Kroner A, Hofstetter HH, Rieckmann P: Frequency analysis of HLA-B7-restricted Epstein-Barr virus-specific cytotoxic T lymphocytes in patients with multiple sclerosis and healthy controls. J Neuroimmunol. 2006, 180: 185-192. 10.1016/j.jneuroim.2006.08.008.
Höllsberg P, Hansen HJ, Haahr S: Altered CD8+ T cell responses to selected Epstein-Barr virus immunodominant epitopes in patients with multiple sclerosis. Clin Exp Immunol. 2003, 132: 137-143. 10.1046/j.1365-2249.2003.02114.x.
Pender MP, Csurhes PA, Lenarczyk A, Pfluger CMM, Burrows SR: Decreased T cell reactivity to Epstein-Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009, 80: 498-505. 10.1136/jnnp.2008.161018.
Pender MP, Csurhes PA, Pfluger CMM, Burrows SR: CD8 T cell deficiency impairs control of Epstein-Barr virus and worsens with age in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011
Cepok S, Zhou D, Srivastava R, Nessler S, Stei S, Büssow K, Sommer N, Hemmer B: Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005, 115: 1352-1360.
Craig JC, Hawkins SA, Swallow MW, Lyttle JA, Patterson VH, Merrett JD, Haire M: Subsets of T lymphocytes in relation to T lymphocyte function in multiple sclerosis. Clin Exp Immunol. 1985, 61: 548-555.
Lindsey JW, Hatfield LM: Epstein-Barr virus and multiple sclerosis: cellular immune response and cross-reactivity. J Neuroimmunol. 2010, 229: 238-242. 10.1016/j.jneuroim.2010.08.009.
Li F, Linan MJ, Stein MC, Faustman DL: Reduced expression of peptide-loaded HLA class I molecules on multiple sclerosis lymphocytes. Ann Neurol. 1995, 38: 147-154. 10.1002/ana.410380205.
Polman CH, Reingold SC, Edan G, Filippi M, Hartung H-P, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS: Diagnostic criteria for multiple sclerosis: 2005 Revisions to the "McDonald Criteria". Ann Neurol. 2005, 58: 840-846. 10.1002/ana.20703.
Kurtzke JF: Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983, 33: 1444-1452.
Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, Achiti I, Confavreux C, Coustans M, le Page E, Edan G, McDonnell GV, Hawkins S, Trojano M, Liguori M, Cocco E, Marrosu MG, Tesser F, Leone MA, Weber A, Zipp F, Miterski B, Epplen JT, Oturai A, Sørensen PS, Celius EG, Lara NT, Montalban X, Villoslada P, Silva AM, Marta M, Leite I, Dubois B, Rubio J, Butzkueven H, Kilpatrick T, Mycko MP, Selmaj KW, Rio ME, Sá M, Salemi G, Savettieri G, Hillert J, Compston DA: Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005, 64: 1144-1151. 10.1212/01.WNL.0000156155.19270.F8.
Kouwenhoven M, Teleshova N, Özenci V, Press R, Link H: Monocytes in multiple sclerosis: phenotype and cytokine profile. J Neuroimmunol. 2001, 112: 197-205. 10.1016/S0165-5728(00)00396-9.
Gudgeon NH, Taylor GS, Long HM, Haigh TA, Rickinson AB: Regression of Epstein-Barr virus-induced B-cell transformation in vitro involves virus-specific CD8+ T cells as the principal effectors and a novel CD4+ T-cell reactivity. J Virol. 2005, 79: 5477-5488. 10.1128/JVI.79.9.5477-5488.2005.
Reinherz EL, Weiner HL, Hauser SL, Cohen JA, Distaso JA, Schlossman SF: Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980, 303: 125-129. 10.1056/NEJM198007173030303.
Bach M-A, Phan-Dinh-Tuy F, Tournier E, Chatenoud L, Bach J-F, Martin C, Degos J-D: Deficit of suppressor T cells in active multiple sclerosis. Lancet. 1980, 316: 1221-1223. 10.1016/S0140-6736(80)92480-0.
Compston A: Lymphocyte subpopulations in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1983, 46: 105-114. 10.1136/jnnp.46.2.105.
Antel JP, Peeples DM, Reder AT, Arnason BGW: Analysis of T regulator cell surface markers and functional properties in multiple sclerosis. J Neuroimmunol. 1984, 6: 93-103. 10.1016/0165-5728(84)90030-4.
Thompson AJ, Brazil J, Whelan CA, Martin EA, Hutchinson M, Feighery C: Peripheral blood T lymphocyte changes in multiple sclerosis: a marker of disease progression rather than of relapse?. J Neurol Neurosurg Psychiatry. 1986, 49: 905-912. 10.1136/jnnp.49.8.905.
Trotter JL, Clifford DB, McInnis JE, Griffeth RC, Bruns KA, Perlmutter MS, Anderson CB, Collins KG, Banks G, Hicks BC: Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann Neurol. 1989, 25: 172-178. 10.1002/ana.410250211.
Kreuzfelder E, Shen G, Bittorf M, Scheiermann N, Thraenhart O, Seidel D, Grosse-Wilde H: Enumeration of T, B and natural killer peripheral blood cells of patients with multiple sclerosis and controls. Eur Neurol. 1992, 32: 190-194. 10.1159/000116820.
Michałowska-Wender G, Wender M: Mononuclear subsets in the peripheral blood of multiple sclerosis patients in relation to results of brain gadolinium-enhancing imaging. Folia Neuropathol. 2006, 44: 67-71.
Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L: Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995, 1: 1279-1283. 10.1038/nm1295-1279.
Ferreira MAR, Mangino M, Brumme CJ, Zhao ZZ, Medland SE, Wright MJ, Nyholt DR, Gordon S, Campbell M, McEvoy BP, Henders A, Evans DM, Lanchbury JS, Pereyra F, International HIV Controllers Study, Walker BD, Haas DW, Soranzo N, Spector TD, de Bakker PI, Frazer IH, Montgomery GW, Martin NG: Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am J Hum Genet. 2010, 86: 88-92. 10.1016/j.ajhg.2009.12.008.
Jabs DA, Arnett FC, Bias WB, Beale MG: Familial abnormalities of lymphocyte function in a large Sjögren's syndrome kindred. J Rheumatol. 1986, 13: 320-326.
Johnston C, Alviggi L, Millward BA, Leslie RDG, Pyke DA, Vergani D: Alterations in T-lymphocyte subpopulations in type 1 diabetes. Exploration of genetic influence in identical twins. Diabetes. 1988, 37: 1484-1488. 10.2337/diabetes.37.11.1484.
Johansen M, Elling P, Elling H, Olsson A: A genetic approach to the aetiology of giant cell arteritis: depletion of the CD8+ T-lymphocyte subset in relatives of patients with polymyalgia rheumatica and arteritis temporalis. Clin Exp Rheumatol. 1995, 13: 745-748.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/11/95/prepub
Acknowledgements
This study was supported by project grants from Multiple Sclerosis Research Australia. We are grateful to Dr Kerryn Green, Kaye Hooper and Bernie Gazzard for assistance in clinically assessing patients and in the collection of blood samples and to Dr Peter Baker for statistical advice.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MPP planned the study, recruited and assessed the patients, helped analyze the results, and wrote the manuscript. PAC performed the laboratory studies and analyzed the data. CMMP assisted with the laboratory studies. SRB helped plan the study and analyze the data. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pender, M.P., Csurhes, P.A., Pfluger, C.M. et al. Decreased CD8+T cell response to Epstein-Barr virus infected B cells in multiple sclerosis is not due to decreased HLA class I expression on B cells or monocytes. BMC Neurol 11, 95 (2011). https://doi.org/10.1186/1471-2377-11-95
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-11-95