Abstract
Background
A possible synergic role of serum uric acid (SUA) with thrombolytic therapies is controversial and needs further investigations. We therefore evaluated association of admission SUA with clinical improvement and clinical outcome in patients receiving rt-PA, early admitted patients not receiving rt-PA, and patients admitted after time window for rt-PA.
Methods
SUA levels were obtained at admission and categorized as low, middle and high, based on 33° and 66° percentile values. Patients were categorized as patients admitted within 3 hours of symptom onset receiving rt-PA (rt-PA group), patients admitted within 3 hours of symptom onset not receiving rt-PA (non-rt-PA group), and patients admitted after time window for rt-PA (late group). Short-term clinical improvement was defined as the difference between NIHSS on admission minus NIHSS day 7. Favorable outcome was defined as mRS 0 - 3 and unfavorable outcome as mRS 4 - 6.
Results
SUA measurements were available in 1136 patients. Clinical improvement was significantly higher in patients with high SUA levels at admission. After adjustment for possible confounders, SUA level showed a positive correlation with clinical improvement (r = 0.012, 95% CI 0.002-0.022, p = 0.02) and was an independent predictor for favorable stroke outcome (OR 1.004; 95% CI 1.0002-1.009; p = 0.04) only in the rt-PA group.
Conclusions
SUA may not be neuroprotective alone, but may provide a beneficial effect in patients receiving thrombolysis.
Similar content being viewed by others
Background
Serum uric acid (SUA) is a final enzymatic product of purine metabolism [1, 2]. Animal models of acute ischemic stroke (AIS) have shown that SUA may be neuroprotective [3] and may reinforce the benefits of intravenous thrombolysis (rt-PA) [4]. In humans, high SUA may be an independent predictor of better outcome after AIS [5] and represent a consumptive and reproducible antioxidant in AIS [6, 7]. Combined intravenous administration of rt-PA and SUA is safe, prevents an early decline in SUA levels and decreases lipid peroxidation but has not shown any clinical effect [8]. On the other hand, high SUA levels have been also associated with hypertension [9], dyslipidemia, type 2 diabetes [10], kidney disease, cardiovascular and cerebrovascular events [11] and worse functional outcome after AIS [12]. The role of SUA in AIS is therefore still controversial, and a possible synergic role of SUA with thrombolytic therapies needs further investigations.
In the present study we therefore aimed to evaluate the association of admission SUA levels with short-term clinical improvement and short-term clinical outcome in patients receiving rt-PA, patients admitted within 3 hours of symptom onset not receiving rt-PA, and patients admitted after the time window for rt-PA.
Methods
This prospective study was conducted at Haukeland University Hospital, Bergen, Norway, which serves a well-defined population of 235,000 inhabitants. The study was performed from February 2006 to January 2009 as a part of a cohort study (Bergen NORSTROKE Study) in which data are collected from all consecutive ischemic stroke patients admitted to the stroke unit in the Department of Neurology. All patients were managed according to a standard protocol and received standard care as recommended by the European Stroke Organisation [13]. Eligible patients received intravenous rt-PA according to the SITS-MOST protocol [14]. SUA levels, NIH stroke scale (NIHSS) score, serum creatinine levels, systolic and diastolic blood pressure were obtained at admission. Patients with unavailable SUA levels at admission were excluded from the study. Patients were categorized as patients admitted within 3 hours of symptom onset receiving rt-PA (rt-PA group), patients admitted within 3 hours of symptom onset not receiving rt-PA (non-rt-PA group), and patients admitted after time window for rt-PA (late group). Patients were categorized as young (≤ 65 years) and old (> 65 years). NIHSS scores were categorized as mild (< 8), moderate (8 - 14) and severe (> 14) [15]. Short-term clinical progress was defined as the difference between NIHSS on admission (NIHSS0) minus NIHSS at day 7 (NIHSS7) (Δ-NIHSS = NIHSS0 - NIHSS7) [16, 17]. A positive Δ-NIHSS value indicated a clinical improvement whereas a negative value indicated a clinical worsening.
SUA were categorized as low, middle and high, based on 33° and 66° percentile values.
The etiology of stroke was classified as large-artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined etiology and stroke of undetermined etiology based on TOAST criteria [18].
Diabetes mellitus (DM) was defined as treatment with glucose lowering medications or diet before stroke onset. Hypertension (HT) was defined as treatment with antihypertensive drugs before stroke onset. Serum creatinine levels were measured at admission and categorized as normal < 120 μmol/L or high ≥ 120 μmol/L. Previous stroke or TIA were registered. Seven days after stroke onset, functional outcome was assessed by modified Rankin scale (mRS) [19]. Assessment was done by a trained stroke nurse. Favorable outcome was defined as mRS 0 - 3 and unfavorable outcome as mRS 4 - 6 [20]. The study was approved by the local research ethics committee and informed consent was obtained from all patients as part of a prospective study protocol.
Statistics
Chi-square test, Student's t-test, and analysis of variance (ANOVA) were used as appropriate. Age, sex, systolic blood pressure at admission, serum creatinine, pre-existing diabetes were considered as possible confounding factors of SUA level. Multiple linear regression was used to estimate the relationship between SUA and Δ-NIHSS. Age, sex, systolic blood pressure at admission, serum creatinine, pre-existing diabetes and admission NIHSS score were included as other exposure variables. Logistic regression analysis was performed to determine factors that could be considered independent predictors for stroke outcome. Significance was set at p < 0.05. The analysis was performed with the software 'STATA/SE 11.0 for Windows'.
Results
During the study period, 1224 patients with ischemic stroke were admitted to our department. SUA measurements were available in 1136 patients. Missing values were due to technical error and may be called protocol violations. Basic characteristics of the study population are shown in Table 1. SUA levels related to demographics and risk factors of the population groups are shown in Table 2.
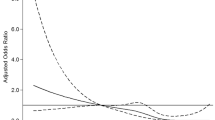
Among the three groups, the rt-PA group had the largest short-term clinical improvement (Table 1). Within this group, the improvement was significantly larger in patients with high SUA levels at admission (Table 3). Multiple linear regression that included patients characteristics (age, gender), stroke characteristics (NIHSS score at admission) and possible confounding factors for SUA levels (serum creatinine, systolic blood pressure at admission) as other exposure variables, showed a positive correlation between admission SUA levels and Δ-NIHSS only in the rt-PA group (r = 0.012, 95% CI 0.002-0.022, p = 0.02), Figure 1.
Correlation between SUA levels and Δ-NIHSS. Age, gender, NIHSS0, serum creatinine and admission systolic blood pressure are included as other exposure variables in the multiple linear regression model. Δ-NIHSS: NIHSS on admission (NIHSS0) minus NIHSS at day 7 (NIHSS7) rt-PA: r = 0.012, 95% CI 0.002-0.022, p = 0.02 Early, no rt-PA: r = 0.012, 95% CI -0.004-0.007, p = 0.7 Late: r = -0.003, 95% CI -0.006-0.0002, p = 0.07.
Stepwise logistic regression analysis including the same covariates as the multiple linear regression, identified SUA levels as predictor for favorable stroke outcome (mRS 0-3) only in the rt-PA group (OR 1.004; 95% CI 1.0002-1.009; p = 0.04). Young age and low NIHSS score at admission were predictors for better stroke outcome in all the three groups.
Discussion
To be effective, neuroprotective agents should act in the acute phase of ischemic stroke, before neuronal death has occurred. We therefore focused our study on SUA levels measured within the first 3 hours after stroke onset. Antioxidant agents have so far shown to be effective only in animal models of AIS, but not in humans [21]. Potential factors hindering translation of animal models success into clinical practice may be patient heterogeneity, inappropriate dose, and wide time window [22]. SUA is a natural antioxidant present in biological fluids throughout the body, and appears to be the major endogenous antioxidant [23, 24]. However, it has been controversial whether SUA is neuroprotective or neurotoxic. Recently, it has been proposed that SUA may show both anti- and pro-oxidant properties depending on levels of other antioxidants, levels of oxidative stress and time of interaction with the target tissues [25], and that the balance between the anti- and pro-oxidant properties shifts in favour of neuroprotection in conditions of extraordinary oxidative stress such as AIS [24]. In our study, SUA level did not show any correlation with clinical improvement nor association with better clinical outcome in early patients not receiving rt-PA. Early studies investigating a possible association between SUA and clinical outcome showed controversial results [5, 12]. However, a potential limitation of these studies may be the wide time window for SUA measurements (e.g up to 48 and 72 hours). Moreover, the study which showed that SUA level predicts poor outcome after AIS [12] included also patients with TIA and intracranial hemorrhage, and did not specify whether a percentage of AIS patients received thrombolytic therapy.
Thrombolysis may lead to recanalization which can salvage viable tissue in the penumbral zone, and is currently the only approved treatment for acute stroke. Dual therapy with rt-PA and neuroprotective agents has shown promising results only in model of AIS [26, 27]. Combined administration of uric acid and rt-PA to adult rats twenty minutes after the induction of ischemia caused a significant reduction in infarct volume and a significant lower neurological impairment [3, 4]. In humans, higher SUA levels in AIS patients receiving rt-PA have been associated with better outcome at day 90 and smaller infarct volume [28]. In agreement with this, our study shows that high SUA is independently associated with higher short-term clinical improvement and better short-term clinical outcome in rt-PA patients.
During the first hours of AIS, reactive oxygen species (ROS) produced by mitochondria play a central pathogenetic role in neuronal death. A further burst of ROS occurs during the early tissue reperfusion induced by recanalization [29]. Serial SUA measurements have shown that SUA is consumed during the first six hours after stroke [6, 7], suggesting that SUA represents a consumptive and reproducible antioxidant in AIS. SUA may therefore supply an additional beneficial effect to rt-PA by acting as a scavenger engulfing ROS released during the early recanalization induced by rt-PA. Even though we do not have data on recanalization rate, this is likely to be higher in the rt-PA group than in the early non-rt-PA group.
Conclusion
In our study high SUA was independently associated with higher short-term clinical improvement and better short-term clinical outcome in rt-PA patients whereas no association was found in early patients not receiving rt-PA or in late patients. SUA may not achieve clinical effect alone, but may provide an additional beneficial effect in patients receiving recanalizing therapies.
References
Becker BF: Towards the physiological function of uric acid. Free Radic Biol Med. 1993, 14 (6): 615-631. 10.1016/0891-5849(93)90143-I.
Sinha S, Singh SN, Ray US: Total antioxidant status at high altitude in lowlanders and native highlanders: role of uric acid. High Alt Med Biol. 2009, 10 (3): 269-274. 10.1089/ham.2008.1082.
Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP: Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998, 53 (5): 613-625. 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1.
Romanos E, Planas AM, Amaro S, Chamorro A: Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007, 27 (1): 14-20. 10.1038/sj.jcbfm.9600312.
Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH: Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002, 33 (4): 1048-1052. 10.1161/hs0402.105927.
Hong JM, Bang OY, Chung CS, Joo IS, Gwag BJ, Ovbiagele B: Influence of recanalization on uric acid patterns in acute ischemic stroke. Cerebrovasc Dis. 2010, 29 (5): 431-439. 10.1159/000289346.
Brouns R, Wauters A, Van De Vijver G, De Surgeloose D, Sheorajpanday R, De Deyn PP: Decrease in uric acid in acute ischemic stroke correlates with stroke severity, evolution and outcome. Clin Chem Lab Med. 2010, 48 (3): 383-390. 10.1515/CCLM.2010.065.
Amaro S, Soy D, Obach V, Cervera A, Planas AM, Chamorro A: A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke. 2007, 38 (7): 2173-2175. 10.1161/STROKEAHA.106.480699.
Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M: Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease?. Hypertension. 2003, 41 (6): 1183-1190. 10.1161/01.HYP.0000069700.62727.C5.
Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K: Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003, 18 (6): 523-530.
Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med. 2008, 359 (17): 1811-1821. 10.1056/NEJMra0800885.
Weir CJ, Muir SW, Walters MR, Lees KR: Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003, 34 (8): 1951-1956. 10.1161/01.STR.0000081983.34771.D2.
Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008, 25 (5): 457-507.
Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, et al: Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007, 369 (9558): 275-282. 10.1016/S0140-6736(07)60149-4.
Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al: Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989, 20 (7): 864-870. 10.1161/01.STR.20.7.864.
Prabhakaran S, Chen M, Choi JH, Mangla S, Lavine SD, Pile-Spellman J, Meyers PM, Chong JY: Major neurologic improvement following endovascular recanalization therapy for acute ischemic stroke. Cerebrovasc Dis. 2008, 25 (5): 401-407. 10.1159/000121340.
Nam HS, Lee KY, Han SW, Kim SH, Lee JY, Ahn SH, Kim DJ, Kim DI, Nam CM, Heo JH: Prediction of long-term outcome by percent improvement after the first day of thrombolytic treatment in stroke patients. J Neurol Sci. 2009, 281 (1-2): 69-73. 10.1016/j.jns.2009.02.365.
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993, 24 (1): 35-41. 10.1161/01.STR.24.1.35.
Banks JL, Marotta CA: Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007, 38 (3): 1091-1096. 10.1161/01.STR.0000258355.23810.c6.
Sulter G, Steen C, De Keyser J: Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999, 30 (8): 1538-1541. 10.1161/01.STR.30.8.1538.
Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, et al: NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008, 39 (6): 1751-1758. 10.1161/STROKEAHA.107.503334.
Cheng YD, Al-Khoury L, Zivin JA: Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004, 1 (1): 36-45. 10.1602/neurorx.1.1.36.
Ryan M, Grayson L, Clarke DJ: The total antioxidant capacity of human serum measured using enhanced chemiluminescence is almost completely accounted for by urate. Ann Clin Biochem. 1997, 34 (Pt 6): 688-689.
Proctor PH: Uric acid: neuroprotective or neurotoxic?. Stroke. 2008, 39 (5): e88-10.1161/STROKEAHA.107.513242. author reply e89
Sautin YY, Johnson RJ: Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008, 27 (6): 608-619. 10.1080/15257770802138558.
Zivin JA, Mazzarella V: Tissue plasminogen activator plus glutamate antagonist improves outcome after embolic stroke. Arch Neurol. 1991, 48 (12): 1235-1238.
Bowes MP, Rothlein R, Fagan SC, Zivin JA: Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995, 45 (4): 815-819.
Amaro S, Urra X, Gomez-Choco M, Obach V, Cervera A, Vargas M, Torres F, Rios J, Planas AM, Chamorro A: Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke. 2011, 42 (1 Suppl): S28-32.
Becker LB: New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004, 61 (3): 461-470. 10.1016/j.cardiores.2003.10.025.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/11/114/prepub
Acknowledgements
This study was supported by The Norwegian Foundation for Health and Rehabilitation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NL was involved in the conception and design of the study, acquisition, analysis and interpretation of data, and drafting the manuscript. HN was involved in the conception and design of the study, and acquisition, analysis and interpretation of data. JB was involved in the conception and design of the study, and acquisition, analysis and interpretation of data. TTI was involved in acquisition of data and drafting the manuscript. UWA was involved in the conception and design of the study, acquisition, analysis and interpretation of data and drafting the manuscript. LT was involved in the conception and design of the study, acquisition, analysis and interpretation of data, and drafting the manuscript. All authors participated in the revision of the manuscript for important intellectual content and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Logallo, N., Naess, H., Idicula, T.T. et al. Serum uri acid: neuroprotection in thrombolysis. The Bergen NORSTROKE study. BMC Neurol 11, 114 (2011). https://doi.org/10.1186/1471-2377-11-114
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-11-114