Abstract
Background
Anophthalmia and microphthalmia are etiologically and clinically heterogeneous. Lenz microphthalmia is a syndromic form that is typically inherited in an X-linked pattern, though the causative gene mutation is unknown. Townes-Brocks syndrome manifests thumb anomalies, imperforate anus, and ear anomalies. We present a 13-year-old boy with a syndromic microphthalmia phenotype and a clinical diagnosis of Lenz microphthalmia syndrome.
Case Presentation
The patient was subjected to clinical and molecular evaluation, including array CGH analysis. The clinical features included left clinical anophthalmia, right microphthalmia, anteriorly placed anus with fistula, chordee, ventriculoseptal defect, patent ductus arteriosus, posteriorly rotated ears, hypotonia, growth retardation with delayed bone age, and mental retardation. The patient was found to have an approximately 5.6 Mb deletion of 16q11.2q12.1 by microarray based-comparative genomic hybridization, which includes the SALL1 gene, which causes Townes-Brocks syndrome.
Conclusions
Deletions of 16q11.2q12.2 have been reported in several individuals, although those prior reports did not note microphthalmia or anophthalmia. This region includes SALL1, which causes Townes-Brocks syndrome. In retrospect, this child has a number of features that can be explained by the SALL1 deletion, although it is not clear if the microphthalmia is a rare feature of Townes-Brocks syndrome or caused by other mechanisms. These data suggest that rare copy number changes may be a cause of syndromic microphthalmia allowing a personalized genomic medicine approach to the care of patients with these aberrations.
Similar content being viewed by others
Background
Anophthalmia and microphthalmia (A/M) are part of a spectrum of ocular anomalies that includes coloboma. These conditions have a combined incidence of about 2/10,000 births [1–4]. The A/M spectrum may be isolated or syndromic and is etiologically and clinically heterogeneous with substantial phenotypic overlap in many cases. This makes accurate syndrome identification crucial in order to provide families and individuals with appropriate recurrence risk counseling.
One-third of individuals with A/M have associated malformations [4]. Both genetic and environmental causes of A/M have been noted. Mutations causing a phenotype that segregates in an autosomal recessive pattern have been found in PAX6 [5], RAX [6] and CHX10 [7] (MIM 607108, 601881 and 142993, respectively). Mutations in SOX2 (MIM 184429) [8, 9] were recently described in individuals with A/M that segregates in an autosomal dominant pattern. Current estimates suggest that up to 15% of individuals with bilateral A/M have a mutation in SOX2 [9]. Bakrania et al., [10] also found individuals with deletions of SOX2. Mutations in OTX2 (MIM 600037) have been reported in up to 5% of individuals with A/M [9]. Mutations in BCOR (MIM 300485) have been seen in families with X-linked recessive inheritance of a phenotype strikingly similar to Lenz microphthalmia syndrome [11, 12]. Lenz microphthalmia has cardinal manifestations of microphthalmia, growth retardation and limb anomalies [13]. Other malformations thought to be typical of this disorder include microcephaly, cognitive impairment, protuberant ears, missing upper central incisors, syndactyly, and a cylindrical thorax with sloping shoulders. However, it is clear that Lenz microphthalmia is clinically variable and genetically heterogeneous and prior data show that phenotypes compatible with this description map both to Xp and Xq, the former caused by mutations in the BCOR gene (MIM 309800) which causes a phenotype that overlaps extensively with Lenz microphthalmia, and shares the X-linked inheritance pattern in two families [12, 14]. The Xq Lenz locus (MCOPS1 MIM 309800) has not yet been identified [15].
Here we report a patient with syndromic A/M that overlaps with Lenz microphthalmia syndrome (MIM 309800). Molecular evaluation of this patient showed a deletion of 16q, which includes the SALL1 gene, which causes Townes-Brocks syndrome (TBS, MIM 107480). This finding extends the notion of etiologic heterogeneity of syndromic microphthalmia and has implications for the approach to molecular diagnostics for this disorder.
Case Presentation
The patient was born at full term weighing 6 lb 2 oz (~25th centile) and length was 21 in (~75th centile). He was apneic at birth and was intubated and transferred to the NICU. He was weaned from the ventilator at 7 days of age with no complications. Bilateral clinical anophthalmia was diagnosed on clinical examination. A cranial CT scan at one day of age showed mild prominence of the left lateral ventricle. A small cavum septum pellucidum was also noted. A focal area of parenchymal lucency was noted in the left parietal parenchyma. This raised the possibility of an in utero ischemic event. A CT scan of the orbits at 6 days of age showed some orbital contents in the right eye with an abnormal lens and soft tissue overlying the globe, and his clinical diagnosis was modified to left clinical anophthalmia and right microphthalmia. The left globe had a small oval focus of soft tissue, but this tissue could not confidently be determined to be optic. The optic nerves appeared severely hypoplastic. A cranial MRI done at 7 days of age identified absent optic nerves with a small optic chiasm. Renal ultrasound at 2 days of age showed the kidneys measured 3.7 cm bilaterally (normal 4.0-6.0 cm). Echocardiogram identified a small ventriculoseptal defect and patent ductus arteriosus, which both resolved spontaneously. He was also noted to have an anteriorly placed anus with a fistula that was corrected surgically at 2 months of age.
Clinical Genetics evaluation was performed at 5 years 9 months by one of the authors. Additional history at the time of this evaluation showed he failed to meet multiple developmental milestones. His height was 102 cm (<<5th centile; 50th centile for 4 years), weight 29.5 pounds (<<5th centile; ~50th centile for 2 years 6 months), and head circumference was 50 cm (10-25th centile). In addition to the findings described above, he was found to have apparently small, overfolded, and cupped ears, long trunk with narrow shoulders, low set thumbs, and partial cutaneous syndactyly of toes 4-5. Peripheral blood karyotype was performed at the referring institution and was reported as normal, 46,XY. The patient was diagnosed with Lenz microphthalmia syndrome (OMIM 309800 MCOPS1). Complete sequencing of coding exons and flanking introns of BCOR (the single known Lenz syndrome gene) was normal (data not shown).
He was recently re-evaluated at the age of 13 years. He was treated with melatonin to help with his sleep cycle. On examination, his weight was 65 lbs (<<5th centile; ~50th centile for 9 year old), Height 57 in (~10th centile) and head circumference 52.5 cm (~10th centile). In addition to the previously mentioned findings, he had multiple nevi on his chest, back, and limbs. All were 0.3 cm or less. He has made developmental progress but is in special education. Sequencing of SOX2 was normal (data not shown).
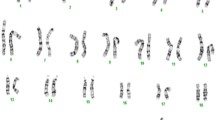
The array platform was of the oligonucleotide CGH type with average genome spacing of 37 kb and targeted probes in candidate gene regions with 5-20 kb probe spacing (for details see http://www.genedx.com/site/genomedx). Hybridization was performed on DNA isolated from peripheral blood leukocytes as previously described [16]. This assay identified an approximately 5.6 Mb deletion of chromosome 16. The deletion likely has a minimum boundary size of Chr16:45,018,886 - 50,571,154 (Human Genome Build 36) and includes 26 known genes (Table 1). This deletion was not present in the parents. The deletion of SALL1 was confirmed by a MLPA assay using the SALSA MLPA kit P180 (MRC Holland, data not shown). Assessment of 6 STRP markers on chromosome 16 showed inheritance of alleles consistent with biologic parentage. The proband is apparently homo/hemizygous at marker D16S3396 (Chr16:49,749,809 - 49,750,146) and does not share an allele with the mother suggesting the deletion occurred on the maternal chromosome. We conclude that this variant is de novo in the proband.
Conclusions
We report a boy with microdeletion of 16q11.2q12.1 identified by array CGH. Microdeletion of 16q11.2q12.2 has been reported as an emerging microdeletion syndrome [17]. That report includes two patients with microdeletions of 16q that overlap the deletion seen in the patient reported here. All three patients share similar centromeric endpoints (as defined by the last centromeric oligonucleotide detected as a single-copy) and two of the three patients share similar telomeric endpoints. The deletion in Patient 1 in Ballif et al [17] extended an additional 1.4 Mb towards the telomere when compared to the present patient. Other patients with deletions of 16q11q12 have been reported with limited molecular data (Table 2).
The deleted region in the patient reported here includes 26 genes, including three recognized disease-causing genes (Table 1). This list includes the SALL1 gene, which when mutated causes Townes-Brocks syndrome [18]. Individuals with whole gene deletions have a milder phenotype than those who have dominant negative point mutations [19]. The classic phenotype for TBS includes imperforate anus, dysplastic ears with hearing impairment, and thumb malformations, although many affected patients do not have the typical phenotypic features. Less frequent manifestations of TBS include renal, heart, foot, and genitourinary anomalies, and mental retardation. This patient has a number of phenotypic manifestations that are not typical of TBS including A/M, cognitive impairment, borderline small head size, growth retardation, abnormal body habitus, and relatively normal thumbs. According to Kohlhase, the clinical diagnosis of TBS requires two of:
* Imperforate anus
* Dysplastic ears (overfolded superior helices, microtia)
* Typical thumb malformations (preaxial polydactyly, triphalangeal thumbs, hypoplastic thumbs) without shortening of the radius http://www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests
The present patient only has one of these manifestations and therefore does not meet these stringent clinical criteria, though as noted above, the phenotype is acknowledged to be variable. One may speculate that an anterior-placed anus with a fistula is reminiscent of TBS, however it does not formally meet the criteria.
In contrast, the classical manifestations of Lenz microphthalmia are microphthalmia, growth retardation, and ear anomalies, all of which this patient manifested [13]. Clearly, this triad is not specific for Lenz microphthalmia and it is reported that > 90% have cognitive impairment [20], as seen in the present patient. A variety of skeletal and limb manifestations have been reported, although frequency data are not available.
Although some of the clinical features of the present patient overlap with the patients previously reported with 16q11.2 deletions and patients with TBS, the patient reported here had a major anomaly, A/M, which is clearly atypical for either of these molecular lesions. Microphthalmia has been reported in a pair of twins with TBS and a point mutation in SALL1 [21] and a patient from the pre-molecular era with a clinical phenotype consistent with TBS [22]. There are several possible hypotheses to explain this observation. First, it is possible that A/M is a very uncommon manifestation of TBS. Insofar as the twins reported by Botzenart et al are concerned, it is remarkable that although both twins had unilateral microphthalmia, neither their TBS-affected siblings nor their TBS-affected father had microphthalmia. It is reported that microphthalmia is two to five times more common in twins than in singletons [23, 24], which limits the utility of this case in assigning causality of the microphthalmia in this pair of twins to the SALL1 mutation. Second it is possible that the deleted region in the patient reported here includes a gene, that when deleted, leads to A/M but with reduced penetrance. The prior report of a single patient with the co-occurrence of TBS and microphthalmia is intriguing [22] in this regard. As this patient has not be characterized molecularly and is now deceased (A.R. Cooper, personal communication), we cannot determine if that child had microphthalmia and TBS because of a 16q deletion or that s/he had a point mutation in SALL1 and microphthalmia is a rare manifestation of TBS. The third possibility is that in the present patient and the patient reported by Fraser and Cooper, [22] the 16q11.2q12.2 deletion is in trans with another variant, likely to be uncommon, that leads to a null for a putative A/M gene in this interval. It will be important to analyze additional cases with deletions of this region to distinguish these two hypotheses.
The deletion region also includes the genes NOD2 associated with Blau syndrome (OMIM 266600) [25] and CYLD associated with Brooke-Spiegler syndrome (MIM 605041) [26]. Missense mutations in NOD2 cause Blau syndrome and an increased susceptibility to Crohn's disease (MIM 266600) [27]. Blau syndrome is inherited in an autosomal dominant pattern and characterized by early-onset granulomatous arthritis, rashes, and camptodactyly. Ocular manifestations include uveitis, glaucoma, retinal detachment, and cataract [28, 29]. No published reports of A/M and Blau syndrome were identified (negative results from PubMed using the two search strings: "anophth* AND Blau" and "microphth* AND Blau").
Heterozygous loss of function mutations in CYLD cause Brooke-Spiegler syndrome, which is characterized by a number of rare skin appendage tumors such as cylindroma, trichoepithelioma, and spiradenoma [30]. Early detection and aggressive treatment can prevent the significant deformity that can be caused by these tumors. These data suggest that the molecular delineation of this deletion in this patient present an opportunity to provide presymptomatic care that will significantly ameliorate the morbidity of this syndrome.
The patient reported here shares some clinical findings with the two previously reported patients [17] with deletion 16q11.2;12.2 namely; ear anomalies, toe abnormalities, hypotonia, and significant developmental delay. Interestingly, Patient 2 from Ballif et al., [17] was reported to have scattered pigmentary nevi, which was also found in the patient reported here.
We conclude from these data that the pleiotropic syndrome in this patient is caused by the deletion, which was detected by array CGH. The phenotype in this patient has some similarities to Townes-Brocks syndrome and to Lenz microphthalmia. The other suggestion from this report is that array CGH analysis should be considered in all patients with syndromic A/M. This is in addition to previous recommendations that such patients undergo chromosome analysis and SOX2 mutation analysis [9, 10].
Consent
The parent of this minor patient has given written consent for the report to be published. The patient was also consented to a clinical research protocol reviewed and approved by the NHGRI IRB.
Abbreviations
- A/M:
-
anophthalmia or microphthalmia
- CGH:
-
comparative genomic hybridization
- aCGH:
-
array CGH
- TBS:
-
Townes Brocks syndrome
References
Clementi M, Tenconi R, Bianchi F, Botto L, Calabro A, Calzolari E, Cianciulli D, Mammi I, Mastroiacovo P, Meli P, Spagnolo A, Turolla L, Volpato S: Congenital eye malformations: a descriptive epidemiologic study in about one million newborns in Italy. Birth Defects Orig Artic Ser. 1996, 30: 413-424.
Kallen B, Robert E, Harris J: The descriptive epidemiology of anophthalmia and microphthalmia. Int J Epidemiol. 1996, 25: 1009-1016. 10.1093/ije/25.5.1009.
Dolk H, Busby A, Armstrong BG, Walls PH: Geographical variation in anophthalmia and microphthalmia in England, 1988-94. BMJ. 1998, 317: 905-909. discussion 910.
Morrison D, FitzPatrick D, Hanson I, Williamson K, van Heyningen V, Fleck B, Jones I, Chalmers J, Campbell H: National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology. Journal of Medical Genetics. 2002, 39: 16-22. 10.1136/jmg.39.1.16.
Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL: PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genetics. 1994, 7: 463-471. 10.1038/ng0894-463.
Voronina VA, Kozhemyakina EA, O'Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH: Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Human Molecular Genetics. 2004, 13: 315-322. 10.1093/hmg/ddh025.
Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, Kocak-Altintas A, Sowden JC, Traboulsi E, Sarfarazi M, McInnes RR: Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nature Genetics. 2000, 25: 397-401. 10.1038/78071.
Male A, Davies A, Bergbaum A, Keeling J, FitzPatrick D, Mackie Ogilvie C, Berg J: Delineation of an estimated 6.7 MB candidate interval for an anophthalmia gene at 3q26.33-q28 and description of the syndrome associated with visible chromosome deletions of this region. European Journal of Human Genetics. 2002, 10: 807-812. 10.1038/sj.ejhg.5200890.
Ragge NK, Lorenz B, Schneider A, Bushby K, de Sanctis L, de Sanctis U, Salt A, Collin JR, Vivian AJ, Free SL, Thompson P, Williamson KA, Sisodiya SM, van Heyningen V, Fitzpatrick DR: SOX2 anophthalmia syndrome. Am J Med Genet A. 2005, 135: 1-7. discussion 8.
Bakrania P, Robinson DO, Bunyan DJ, Salt A, Martin A, Crolla JA, Wyatt A, Fielder A, Ainsworth J, Moore A, Read S, Uddin J, Laws D, Pascuel-Salcedo D, Ayuso C, Allen L, Collin JR, Ragge NK: SOX2 anophthalmia syndrome: 12 new cases demonstrating broader phenotype and high frequency of large gene deletions. Br J Ophthalmol. 2007, 91: 1471-1476. 10.1136/bjo.2007.117929.
Hilton E, Johnston J, Whalen S, Okamoto N, Hatsukawa Y, Nishio J, Kohara H, Hirano Y, Mizuno S, Torii C, et al: BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. European Journal of Human Genetics. 2009
Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, Hadley DW, Tifft C, Zhang L, Wilkie AO, Smagt van der JJ, Gorlin RJ, Burgess SM, Bardwell VJ, Black GC, Biesecker LG: Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet. 2004, 36: 411-416. 10.1038/ng1321.
Jones KL: Smith's Recognizable Patterns of Human Malformation. 2006, Philadelphia: Elsevier Saunders, 6
Hilton E, Johnston J, Whalen S, Okamoto N, Hatsukawa Y, Nishio J, Kohara H, Hirano Y, Mizuno S, Torii C, et al: BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. European Journal of Human Genetics. 2009, 17: 1325-1335. 10.1038/ejhg.2009.52.
Graham CA, Redmond RM, Nevin NC: X-linked clinical anophthalmos. Localization of the gene to Xq27-Xq28. Ophthalmic Paediatr Genet. 1991, 12: 43-48. 10.3109/13816819109023084.
Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, Mottron L, Champagne N, Lafreniere RG, Hamdan FF, Joober R, Fombonne E, Marineau C, Cossette P, Dube MP, Haghighi P, Drapeau P, Barker PA, Carbonetto S, Rouleau GA: Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Human Molecular Genetics. 2008, 17: 3965-3974. 10.1093/hmg/ddn300.
Ballif BC, Theisen A, McDonald-McGinn DM, Zackai EH, Hersh JH, Bejjani BA, Shaffer LG: Identification of a previously unrecognized microdeletion syndrome of 16q11.2q12.2. Clinical Genetics. 2008, 74: 469-475. 10.1111/j.1399-0004.2008.01094.x.
Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W: Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nature Genetics. 1998, 18: 81-83. 10.1038/ng0198-81.
Borozdin W, Steinmann K, Albrecht B, Bottani A, Devriendt K, Leipoldt M, Kohlhase J: Detection of heterozygous SALL1 deletions by quantitative real time PCR proves the contribution of a SALL1 dosage effect in the pathogenesis of Townes-Brocks syndrome. Human Mutation. 2006, 27: 211-212. 10.1002/humu.9396.
Gorlin RJ, Cohen MM, Hennekam RCM: Syndromes of the head and neck. 2001, Oxford: Oxford University Press, 4
Botzenhart EM, Bartalini G, Blair E, Brady AF, Elmslie F, Chong KL, Christy K, Torres-Martinez W, Danesino C, Deardorff MA, Fryns JP, Marlin S, Garcia-Minaur S, Hellenbroich Y, Hay BN, Penttinen M, Shashi V, Terhal P, Van Maldergem L, Whiteford ML, Zackai E, Kohlhase J: Townes-Brocks syndrome: twenty novel SALL1 mutations in sporadic and familial cases and refinement of the SALL1 hot spot region. Human Mutation. 2007, 28: 204-205. 10.1002/humu.9476.
Fraser F, Cooper A: Micropthalmia as part of the townes syndrome. Proceedings of the Greenwood Genetics Center. 1985, 4: 129-
Li SJ, Ford N, Meister K, Bodurtha J: Increased risk of birth defects among children from multiple births. Birth Defects Res A Clin Mol Teratol. 2003, 67: 879-885. 10.1002/bdra.10093.
Shaw GM, Carmichael SL, Yang W, Harris JA, Finnell RH, Lammer EJ: Epidemiologic characteristics of anophthalmia and bilateral microphthalmia among 2.5 million births in California, 1989-1997. Am J Med Genet A. 2005, 137: 36-40.
Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP: CARD15 mutations in Blau syndrome. Nature Genetics. 2001, 29: 19-20. 10.1038/ng720.
Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, Ouweland van Den A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S: Identification of the familial cylindromatosis tumour-suppressor gene. Nature Genetics. 2000, 25: 160-165. 10.1038/76006.
Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G: Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001, 411: 599-603. 10.1038/35079107.
Blau EB: Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985, 107: 689-693. 10.1016/S0022-3476(85)80394-2.
Pastores GM, Michels VV, Stickler GB, Su WP, Nelson AM, Bovenmyer DA: Autosomal dominant granulomatous arthritis, uveitis, skin rash, and synovial cysts. J Pediatr. 1990, 117: 403-408. 10.1016/S0022-3476(05)81080-7.
Scheinfeld N, Hu G, Gill M, Austin C, Celebi JT: Identification of a recurrent mutation in the CYLD gene in Brooke-Spiegler syndrome. Clin Exp Dermatol. 2003, 28: 539-541. 10.1046/j.1365-2230.2003.01344.x.
Hoo JJ, Lowry RB, Lin CC, Haslam RH: Recurrent de novo interstitial deletion of 16q in two mentally retarded sisters. Clinical Genetics. 1985, 27: 420-425.
Knoblauch H, Thiel G, Tinschert S, Korner H, Tennstedt C, Chaoui R, Kohlhase J, Dixkens C, Blanck C: Clinical and molecular cytogenetic studies of a large de novo interstitial deletion 16q11.2-16q21 including the putative transcription factor gene SALL1. Journal of Medical Genetics. 2000, 37: 389-392. 10.1136/jmg.37.5.389.
Krauss CM, Caldwell D, Atkins L: Interstitial deletion and ring chromosome derived from 16q. Journal of Medical Genetics. 1987, 24: 308-312. 10.1136/jmg.24.5.308.
Schuffenhauer S, Callen DF, Seidel H, Shen Y, Lederer G, Murken J: De novo interstitial deletion 16(q12.1q13) of paternal origin in a 10-year-old boy. Clinical Genetics. 1992, 42: 246-250.
Callen DF, Eyre H, Lane S, Shen Y, Hansmann I, Spinner N, Zackai E, McDonald-McGinn D, Schuffenhauer S, Wauters J, et al: High resolution mapping of interstitial long arm deletions of chromosome 16: relationship to phenotype. Journal of Medical Genetics. 1993, 30: 828-832. 10.1136/jmg.30.10.828.
Matthaei A, Werner W, Gerlach EM, Koerner U, Tinschert S, Nitz I, Herr A, Rump A, Bartsch O, Hinkel KG, Schrock E, Oexle K: Small reciprocal insertion detected by spectral karyotyping (SKY) and delimited by array-CGH analysis. Eur J Med Genet. 2005, 48: 328-338. 10.1016/j.ejmg.2005.04.024.
Elder FF, Ferguson JW, Lockhart LH: Identical twins with deletion 16q syndrome: evidence that 16q12.2-q13 is the critical band region. Human Genetics. 1984, 67: 233-236. 10.1007/BF00273010.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/10/137/prepub
Acknowledgements
Funding for ASS and TMB was provided by the Albert B. Millett Memorial Fund, A Mellon Mid-Atlantic Charitable Trust, Rae S. Uber Trust, and the Gustavus and Louise Pfeiffer Research Foundation. Funding for JJJ, DN, and LGB was provided by the Intramural Research Program of the National Human Genome Research Institute of the National Institutes of Health. The authors are grateful to the family for their support of this description of their son and their support of the International Anophthalmia Children's Network. Aradhya Swaroop, Ph.D., of GeneDx Corp. provided information on the array platform.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TMB wrote the first draft of the manuscript. ASS and DN examined the patient and edited the manuscript. JJJ designed, interpreted, and performed some of the molecular evaluations, reviewed the literature, and wrote significant portions of the manuscript. LGB designed and interpreted some of the molecular evaluations, examined the patient, and wrote significant portions of the manuscript. All authors have reviewed and approved the submission of the manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bardakjian, T.M., Schneider, A.S., Ng, D. et al. Association of a de novo16q copy number variant with a phenotype that overlaps with Lenz microphthalmia and Townes-Brocks syndromes. BMC Med Genet 10, 137 (2009). https://doi.org/10.1186/1471-2350-10-137
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-10-137