Abstract
Background
Commercial diagnostics are commonly used to identify gram-positive bacteria. Errors have been reported mostly at the species level. We have found certain phenotypic criteria used in API systems which significantly misidentify Leuconostoc, an emerging human pathogen, at the genus level. We also attempt to find practical, conventional phenotypic assays for accurate identification of this group of bacteria.
Methods
Clinical isolates of catalase-negative, gram-positive coccoid or coccobacillary bacteria with non-β hemolysis in our institute during 1997–2004 were subject to an identification aid by API 20 STREP, following the instruction manual, as an aid to conventional phenotypic tests. Those identified as Leuconostoc by API 20 STREP were re-examined by the same kit and also by API 50 CHL according to the instruction manuals, by our Leuconostoc conventional phenotypic assays, by Leuconostoc- and Lactobacillus-specific PCR's, and, where possible, by 16S rDNA sequence analysis. In addition, catalase-negative gram-positive isolates during 2005–2006 which were resistant to vancomycin at high levels were also evaluated by the same phenotypic and genotypic assays.
Results
Out of several thousands of clinical gram-positive isolates, 26 catalase negative gram-positive isolates initially identified as Leuconostoc by API 20 STREP and 7 vancomycin-resistant gram-positive catalase-negative bacteria entered the study. 11 out of the 26 isolates and all the 7 isolates were identified as Leuconostoc by API 20 STREP. Only 5 isolates, however, were confirmed by both genotypic and all defined conventional phenotypic criteria. API 50 CHL also failed to reliably provide accurate identification of Leuconostoc. We have identified key problem tests in API 20 STREP leading to misidentification of the bacteria. A simple, conventional set of phenotypic tests for Leuconostoc identification is proposed.
Conclusion
The current API systems cannot accurately identify Leuconostoc. Identification of vancomycin-resistant, catalase-negative gram-positive bacteria should be performed by a few practical phenotypic assays, with assistance of genotypic assays where available.
Similar content being viewed by others
Background
Leuconostoc is a gram-positive coccoid or coccobacillary emerging human pathogen found in environment, foods and food products [1]. Risk factors of infection include antibiotic pressure, foreign device, or underlying immune defects. The organism is naturally highly resistant to vancomycin with MIC ≥ 256 μg/ml but could be successfully treated with penicillin with MIC ranging from 0.25 to 1.0 unit/ml. Commercial diagnostics are commonly used to identify gram-positive bacteria, with errors mostly at the species level [2, 3]. Here we report inaccuracies of the Analytical Profile Index systems (API 20 STREP and API 50 CHL, Biomérieux, Inc., Lyon, France) in identifying Leuconostoc at the genus level. We also propose practical methods for clinical bacteriology laboratories to identify this organism.
Methods
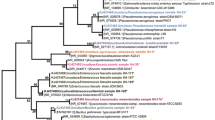
Clinical isolates of catalase negative gram-positive coccoid or coccobacillary pairs and chains with α- or γ-hemolysis in our institute during 1997–2004 were subject to an identification aid by API 20 STREP (bioMérieux, Inc., Lyon, France), following the instruction manual. Those identified as Leuconostoc by API 20 STREP were re-examined by the same kit and by API 50 CHL (bioMérieux, Inc., Lyon, France) according to the instruction manuals, by Leuconostoc conventional phenotypic assays, by Leuconostoc- and Lactobacillus-specific PCR's, and by 16S rDNA sequence analysis as previously described [4]. The 800-bp 16S rDNA fragment corresponds to Escherichia coli positions 10 to 806. The sequencing results were compared with those available in the GenBank, using BLASTN. Criteria for our conventional phenotypic assays for Leuconostoc are catalase-negative gram-positive coccoid or coccobacillary bacteria evaluated after growth in thioglycolate broth at 35°C for 24–48 hours [5], vancomycin MIC ≥ 256 μg/ml by Etest (AB BIODISK, Solna, Sweden), CO2 production from glucose in de Man, Sharp, Rogosa (MRS) broth (Difco, Detroit, MI, USA) with Durham tubes and negative pyrrolidonyl arylamidase (PYR), leucine arylamidase (LAP), and arginine dihydrolase (ADH) [6]. Leuconostoc-specific PCR was performed on all isolates as described [7], with slight primer modifications, as stated below. These modifications were to make the primer sequences most complementary and specific to Leuconostoc strains in GenBank. Forward and reverse primer sequences were 5'-CACAGCGAAAGGTGCTTGCAC-3' and 5'-GATCCATCTCTAGGTGACGCC-3', respectively. To further assess accuracy of the API 20 STREP kit, additional catalase-negative gram-positive coccoid or coccobacillary isolates during 2005–2006 with vancomycin MIC ≥ 256 μg/ml were also evaluated by the same phenotypic and genotypic assays (isolates 31–38 in Table 1). Our gold standard for Leuconostoc identification is that the organisms fulfill both conventional phenotypic criteria and either or both of the genotypic assays (PCR and 16S rDNA sequence analysis). As we suspected that some isolates might have been Lactobacillus, also a lactic acid bacteria with overlapping phenotypes, Lactobacillus-specific PCR was also performed on all isolates as described [8]. PCR using universal primers targeting bacterial 16S rRNA conserved sequences was also performed to ensure template quality. The forward primer Y1 corresponds to positions 20 to 43 in the E. coli 16S rRNA sequence and the reverse primer Y2 corresponds to E. coli positions 361 to 338 [9]; this protocol gave positive results for all isolates in the study. Leuconostoc mesenteroides ATCC 8293, Pediococcus pentosaceus ATCC 33316, Lactobacillus pentosus ATCC 8041 and Lactobacillus plantarum ATCC 14917 served as controls for all assays. This study has been approved by The Institutional Review Board of The Faculty of Medicine, Chulalongkorn University.
Results
Our clinical bacteriology laboratory has a busy service, serving a 1,500-bed university hospital. Out of several thousands of gram-positive bacteria isolated during 1997–2004, 26 catalase-negative gram-positive isolates (isolates 1–26) were initially identified as Leuconostoc by API 20 STREP. 7 catalase-negative gram-positive strains with vancomycin MIC ≥ 256 μg/ml were isolated during 2005–2006 (isolates 31–33 and 35–38). Thus, 33 clinical isolates entered the study. As 16S rDNA sequencing analysis was performed after the other tests, the results were not complete. Some isolates could not be retrieved, and some could not be amplified.
11 isolates of isolates 1–26 were reproducibly identified by API 20 STREP as Leuconostoc (Table 1). Only 3 of the 11 isolates, however, were confirmed by both genotypic and all defined phenotypic criteria (Table 1). 7 catalase-negative gram-positive isolates with vancomycin MIC ≥ 256 μg/ml (isolates 31–33 and 35–38) were all identified as Leuconostoc by API 20 STREP, only 2 of which were confirmed genotypically. API 20 STREP identified Lactobacillus pentosus ATCC 8041 and Pediococcus pentosaceus ATCC 33316 as Leuconostoc with 81.1% and 39.3% identity, respectively. Regarding all 31 non-leuconostoc strains (including reference strains and clinical isolates), API 20 STREP identified 10 of them as Leuconostoc with over 90% identity. 16S rDNA sequencing data were available in 7 of the 10 isolates and all were closely-related Weissella spp. 6 isolates were read as Leuconostoc with 50–90% identity. Two of these were Lactobacillus pentosus ATCC 8041 and Lactobacillus salivarius (isolate 26) and two were Pediococcus (isolates 35 and 38). API 50 CHL identified almost all Leuconostoc correctly, at least to the genus level, except for isolate 3. The kit, however, identified Pediococcus pentosaceus ATCC 33316, 7 out of 8 Weissella spp., all 6 streptococci, and 1 Enterococcus as either Lactobacillus or Leuconostoc. Of all 37 standard and clinical strains, 14 demonstrated at least one discrepant biochemical test results between API 20 STREP and manual phenotypic assays (Table 1). All these belonged to non-leuconostoc isolates and do not affect conventional phenotypic interpretation of these isolates as non-leuconostoc. Identity percentages given by API 20 STREP and API 50 CHL had poor correlations with PCR or phenotypes.
With regard to the 7 isolates of catalase-negative gram-positive bacteria with high-level vancomycin resistance which were all identified as Leuconostoc by API 20 STREP, only 2 of them were confirmed as such by genotypic assays. API 50 CHL correctly identified both isolates as Leuconostoc, one of which was correct at the species level.
Discussion
Commercial diagnostics have been widely used in bacteriology laboratories to identify common organisms, such as Streptococcus, to the species level, or to identify unusual gram-positive organisms in clinical specimens, e.g. Aerococcus, Lactobacillus, and Leuconostoc, among others. Studies illustrating inaccurate identification of various gram-positive pathogens have been published [3, 10–14]. In this study, our purpose is to raise an awareness that Leuconostoc, an emerging human pathogen, can be overdiagnosed by certain commercial diagnostics.
Occasional discrepant results among the same biochemical tests obtained from API 20 STREP and from manual conventional assays are not unexpected, as incomplete agreement of various automated and manual systems have been reported [15–17]. Reproducibility of API 20 STREP for Leuconostoc identification is only moderate in our study. Previous studies have shown higher consistency of bacterial identification by commercial diagnostics [18, 19]. Clinical isolates in our study were initially identified during the time spanning from 1997–2006 and thus repeated API 20 STREP testing was done months or years thereafter. Our lower reproducibility could be, at least partly, due to loss or change in some characteristics by repeated subculture [20, 21].
All 6 standard and clinical Leuconostoc strains were correctly identified by API 20 STREP and 5 by API 50 CHL, at least at the genus level. On the contrary, specificity of Leuconostoc identification by these API kits were only moderate at best. As evidenced by 16S rDNA sequence analysis, most of the isolates misidentified as Leuconostoc by API systems were in the genus Lactobacillus and Weissella, which are closely-related bacteria, followed by Pediococcus, also one of the lactic acid bacteria [6]. API 20 STREP failed to identify these isolates obviously because Lactobacillus and Weissella are not listed in the Identification Table [22]. Even some strains of streptococci and enterococci initially were identified as Leuconostoc. Streptococcus constellatus isolate 19 was misidentified at the species level. API 50 CHL misidentified most Weissella in this study obviously because only one species, Weissella viridescens, is included in the Identification Table of the kit.
Our conventional phenotypic criteria correlated well with Leuconostoc-specific PCR and 16S rDNA sequence analysis in almost all isolates, except for isolates 7 and 36 (Table 1). Isolate 7 was phenotypically compatible with Leuconostoc but negative by PCR. This could be closely-related bacteria which are certain lactobacilli such as L. sanfrancisco, or L. fructosus, or Weissella [23], or some rare Leuconostoc not detected by our PCR protocol. Weisella is a recently-described genus found in a variety of foods. Some of its members used to be Leuconostoc paramesenteroides and heterofermentative lactobacilli. Reliability of the conventional phenotypic criteria in this study is evidenced by the fact that only 1 of the 8 Weissella isolates and none of the 2 Lactobacillus isolates (one identified by 16S rDNA sequencing and both by PCR) was misidentified as Leuconostoc.
The importance of accurate identification of Leuconostoc also needs to be emphasized in the clinical arena. Case reports based on incomplete and/or inappropriate phenotypic criteria with or without assistance of commercial diagnostics are subject to potential errors [24–27], given the fact that Leuconostoc and related bacteria possess overlapping phenotypes. Flawed clinical reports include an incorrect argument that heterofermentative Lactobacillus must hydrolyze arginine [25], while in fact L. sanfrancisco and L. fructosus do not [23], and labeling the organism as Leuconostoc even though the organism was LAP positive [26].
Two major limitations of API 20 STREP are noted. Firstly, the test contains Leuconostoc in its list, while some other medically-important lactic acid bacteria with overlapping phenotypes such as Lactobacillus, Weissella and Pediococcus, are not included. It is of note, however, that, according to the manufacturer, Leuconostoc is a multiple taxon of Leuconostoc and Lactobacillus and if a strain is identified as Leuconostoc, a note "POSSIBILITY OF Lactobacillus spp" is included in the report. Considering Leuconostoc as a multiple taxon of Leuconostoc and Lactobacillus by the manufacturer is not very practical, as Leuconostoc and Lactobacillus are distinct bacteria, microbiologically and clinically. Given that human infections by these lactic acid bacteria are emerging, these organisms could obviously be misidentified as Leuconostoc by API 20 STREP, potentially contributing to cumulative incorrect reporting in medical literature and incorrect understanding of its clinical spectra and epidemiology. Secondly, while clinical isolates of Leuconostoc, as a rule, are LAP and ADH negative, the test lists Leuconostoc as 70% LAP and 10% ADH positive [22]. Appropriate modifications of the kit criteria for Leuconostoc would significantly enhance its accuracy. For clinical laboratories, we propose that all catalase-negative gram-positive coccoid or coccobacillary bacteria with high level of vancomycin resistance (MIC ≥ 256 μg/ml) be tested with the manual phenotypic assays listed in Table 1: Gram's staining of the isolate grown in thioglycolate broth, arginine dihydrolase (ADH), leucine arylamidase (LAP), gas production in MRS broth, and pyrrolidonyl arylamidase (PYR) test. Users of this method are to accept that, even though more accurate than the current API systems, these conventional assays could still occasionally misidentify certain lactobacilli and Weissella as Leuconostoc. This practical guide should minimize avoidable inaccurate identification of this emerging pathogen.
Conclusion
The current API systems, similar to some other commercial identification systems for microorganisms, still need improvement before they can reliably identify certain unusual gram-positive pathogens. They lack specificity in Leuconostoc identification. We propose that, for accuracy and reliability, identification of vancomycin-resistant, catalase-negative gram-positive bacteria be performed by practical, conventional phenotypic assays, with assistance of a genotypic confirmation where available.
References
Horowitz HW, Handwerger S, van Horn KG, Wormser GP: Leuconostoc, an emerging vancomycin-resistant pathogen. Lancet. 1987, 2 (8571): 1329-1330. 10.1016/S0140-6736(87)91217-7.
MacGowan AP, Marshall RJ, Reeves DS: Evaluation of API 20 STREP system for identifying Listeria species. J Clin Pathol. 1989, 42 (5): 548-550. 10.1136/jcp.42.5.548.
Winston LG, Pang S, Haller BL, Wong M, Chambers HF, Perdreau-Remington F: API 20 strep identification system may incorrectly speciate enterococci with low level resistance to vancomycin. Diagn Microbiol Infect Dis. 2004, 48 (4): 287-288. 10.1016/j.diagmicrobio.2003.10.008.
Bosshard PP, Abels S, Zbinden R, Bottger EC, Altwegg M: Ribosomal DNA sequencing for identification of aerobic gram-positive rods in the clinical laboratory (an 18-month evaluation). J Clin Microbiol. 2003, 41 (9): 4134-4140. 10.1128/JCM.41.9.4134-4140.2003.
Facklam R, Hollis D, Collins MD: Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989, 27 (4): 724-730.
Ruoff KL: Aerococcus, Abiotrophia, and Other Infrequently Isolated Aerobic Catalase-Negative, Gram-Positive Cocci. Manual of Clinical Microbiology. Edited by: Murray PR. 2003, Washington, D.C.: ASM Press, 434-444. 8
Yost CK, Nattress FM: The use of multiplex PCR reactions to characterize populations of lactic acid bacteria associated with meat spoilage. Lett Appl Microbiol. 2000, 31 (2): 129-133. 10.1046/j.1365-2672.2000.00776.x.
Dubernet S, Desmasures N, Gueguen M: A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Lett. 2002, 214 (2): 271-275. 10.1111/j.1574-6968.2002.tb11358.x.
Young JP, Downer HL, Eardly BD: Phylogeny of the phototrophic rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J Bacteriol. 1991, 173 (7): 2271-2277.
Hamilton-Miller JM, Shah S: Vancomycin susceptibility as an aid to the identification of lactobacilli. Lett Appl Microbiol. 1998, 26 (2): 153-154. 10.1046/j.1472-765X.1998.00297.x.
Ling JM, Char TS, Cheng AF: Distribution of enterococci in Hong Kong. J Infect. 2002, 45 (4): 257-262. 10.1053/jinf.2002.1054.
Mackey T, Lejeune V, Janssens M, Wauters G: Identification of vancomycin-resistant lactic bacteria isolated from humans. J Clin Microbiol. 1993, 31 (9): 2499-2501.
Velasco D, Perez S, Pena F, Dominguez MA, Cartelle M, Molina F, Moure R, Villanueva R, Bou G: Lack of correlation between phenotypic techniques and PCR-based genotypic methods for identification of Enterococcus spp. Diagn Microbiol Infect Dis. 2004, 49 (3): 151-156. 10.1016/j.diagmicrobio.2004.03.012.
Yeung PS, Sanders ME, Kitts CL, Cano R, Tong PS: Species-specific identification of commercial probiotic strains. J Dairy Sci. 2002, 85 (5): 1039-1051.
Almuzara MN, de Mier C, Barberis CM, Mattera J, Famiglietti A, Vay C: Arcanobacterium hemolyticum: identification and susceptibility to nine antimicrobial agents. Clin Microbiol Infect. 2002, 8 (12): 828-829. 10.1046/j.1469-0691.2002.00535.x.
Murray PR: Standardization of the Analytab Enteric (API 20E) system to increase accuracy and reproducibility of the test for biotype characterization of bacteria. J Clin Microbiol. 1978, 8 (1): 46-49.
Stager CE, Davis JR: Automated systems for identification of microorganisms. Clin Microbiol Rev. 1992, 5 (3): 302-327.
Appelbaum PC, Jacobs MR, Palko WM, Frauenhoffer EE, Duffett A: Accuracy and reproducibility of the IDS rapID STR system for species identification of streptococci. J Clin Microbiol. 1986, 23 (5): 843-846.
Overman TL, Overley JK: Reproducibility of API Staph-Ident system identifications of coagulase-negative staphylococci isolated from blood. J Clin Microbiol. 1990, 28 (11): 2585-2586.
Bryant TN, Lee JV, West PA, Colwell RR: Numerical classification of species of Vibrio and related genera. J Appl Bacteriol. 1986, 61 (5): 437-467.
Katouli M, Kuhn I, Mollby R: Evaluation of the stability of biochemical phenotypes of Escherichia coli upon subculturing and storage. Journal of general microbiology. 1990, 136 (9): 1681-1688.
bioMérieux Technical Library – API 20 Package Insert. [http://www.biomerieux-usa.com/support/techlibrary/api/index.asp]
Carr FJ, Chill D, Maida N: The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002, 28 (4): 281-370. 10.1080/1040-840291046759.
Scano F, Rossi L, Cattelan A, Carretta G, Meneghetti F, Cadrobbi P, Sgarabotto D: Leuconostoc species: a case-cluster hospital infection. Scand J Infect Dis. 1999, 31 (4): 371-373. 10.1080/00365549950163815.
Montejo M, Grande C, Valdivieso A, Testillano M, Minguillan J, Aguirrebengoa K, Ortiz de Urbina J: Abdominal abscess due to leuconostoc species in a liver transplant recipient. J Infect. 2000, 41 (2): 197-198. 10.1053/jinf.2000.0705.
Templin KS, Crook T, Riley T, Whitener C, Aber RC: Spontaneous bacterial peritonitis and bacteremia due to Leuconostoc species in a patient with end-stage liver disease: a case report. J Infect. 2001, 43 (2): 155-157. 10.1053/jinf.2001.0873.
Vagiakou-Voudris E, Mylona-Petropoulou D, Kalogeropoulou E, Chantzis A, Chini S, Tsiodra P, Malamou-Lada E: Multiple liver abscesses associated with bacteremia due to Leuconostoc lactis. Scand J Infect Dis. 2002, 34 (10): 766-767. 10.1080/00365540260348572.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/7/69/prepub
Acknowledgements
The authors thank Dr. Somboon Tanasupawat, Department of Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand, for ATCC bacteria used in the study, Dr. Nattachai Srisawat, Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, for assistance with clinical data collection, Ms. Sivanee Joybai, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand for technical assistance, all during March and April 2004, Chulalongkorn Medical Research Center and Faculty of Medicine OPR 11 Special Laboratories for core molecular facilities throughout the study during 2004–2007. This study was supported by Ratchadapiseksompotch Pilot Study Fund from Faculty of Medicine, Chulalongkorn University and Development Grant for New Faculty/Researchers, Chulalongkorn University in 2004. During the study, Dr. Kulwichit was supported by Faculty Fund, Faculty of Medicine (2004–2007); Research Scholar Support Fund, King Ananda Mahidol Foundation (2004–2005); and Research Scholar Fund, Thailand Research Fund (2004–2005 and 2006–2007).
This work was completed at the Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand, and was presented, in part, at The 44th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, D.C., October 30–November 2, 2004
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
WK conceived of and designed the study, participated in its coordination, analyzed data, and drafted the manuscript. SN participated in coordination of the study, carried out experiments on API systems and conventional assays of bacteria, and assisted with PCR assays. TC performed 16S rDNA sequence analysis. SK carried out all PCR assays. CU performed susceptibility tests. AC helped design and coordinated the study. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kulwichit, W., Nilgate, S., Chatsuwan, T. et al. Accuracies of Leuconostocphenotypic identification: a comparison of API systems and conventional phenotypic assays. BMC Infect Dis 7, 69 (2007). https://doi.org/10.1186/1471-2334-7-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-7-69