Abstract
Background
one of the World Health Organization Millennium Development Goal is to reduce tuberculosis incidence by 2015. However, more of 8.5 million tuberculosis cases have been reported in 2011, with an increase of multidrug-resistant strains. Therefore, the World Health Organization target cannot be reach without the help of a vaccine able to limit the spread of tuberculosis. Nowadays, bacille Calmette-Guérin is the only vaccine available against tuberculosis. It prevents against meningeal and disseminated tuberculosis in children, but its effectiveness against pulmonary form in adolescents and adults is argued.
Method
a systematic review was performed by searches of Pubmed, references of the relevant articles and Aeras and ClinicalTrial.gov websites.
Results
100 articles were included in this review. Three viral vectored booster vaccines, five protein adjuvant booster vaccines, two priming vaccines and two therapeutic vaccines have been analyzed.
Conclusions
Several vaccines are in the pipeline, but further studies on basic research, clinical trial and mass vaccination campaigns are needed to achieve the TB eradication target by 2050.
Similar content being viewed by others
Background
One of the World Health Organization (WHO) Millennium Development goals (MDGs) is to reduce tuberculosis (TB) incidence by 2015. The targets of the Stop TB Partnership, an international coalition designed to coordinate the efforts against TB, are to halve deaths and prevalence of TB by 2015, relative to 1990 levels and to reduce the global incidence of less than one per million population by 2050 [1].
However, WHO reported an estimated 8.7 million new TB cases in 2011, 0.5 million of which occurred among children, and 1.4 million deaths [2]. Among incident cases, 13% are co-infected with human immunodeficiency virus (HIV) [2]. An additional threat to TB control includes the spread of multidrug-resistant (MDR) strains. Among TB-treatment-naïve cases, 3.7% have been estimated to be MDR, percentage that reaches 20% in previously treated TB cases [2].
Considering the above mentioned data and that an active TB case will typically infect 10-15 contacts, advances in diagnostic and therapeutic strategies are not sufficient to achieve the goal of elimination of TB by 2050 [3]. Therefore, new vaccines development is urgently needed for the control of TB. A vaccine will limit initial infection, progression of disease and reactivation of latent TB [4]. Moreover, it can also be an essential tool in tackling the spread of MDR TB.
Nowadays, bacille Calmette-Guérin (BCG) is still the only vaccine available against TB. It was firstly administered as oral vaccine to an infant in 1921 and it is still the only vaccine licensed to prevent TB. It is a live attenuated strain of Mycobacterium bovis, obtained by Albert Calmette and Camille Guérin through 230 in vitro passages over a 13 year-period [5]. Since daughter strains of BCG have been distributed around the world to produce vaccine in manufacture, genetic and antigenic differences have emerged between vaccine strains [6]. Thus, global concerns about safety and efficacy between different strains arose.
BCG is widely used in TB endemic countries, where newborns are immunized as soon as possible after birth with a single intradermal dose [7]. To date, it is estimated that BCG has been administered over 4 billion times and that 120 million children receive BCG every year globally [8].
Meta-analyses of published studies have clearly reported that BCG prevents against meningeal TB and disseminated forms in children [9, 10]. However, randomized clinical trials have reported estimates of protection against pulmonary TB that vary from nil to 80% [6, 11]. Therefore, since pulmonary TB is the most prevalent form of disease in adolescents and adults and the most significant source of TB transmission, BCG is estimated to have little impact in limiting TB spread. Several causes have been considered to explain the variable efficacy of BCG. These includ differences in BCG strains, host genetic and nutritional factors, variable virulence among Mycobacterium tuberculosis (Mtb), interference of environmental mycobacteria, switch to a type 2 immunological response in presence of helminthic infection and variation among trial methods [3, 5, 8, 12, 13]. Recent studies, evaluating interferon-γ (INF-γ) release assay (IGRA) results, suggested that BCG could not only protect against disseminated TB in children, but also against infection [14].
Several studies demonstrated that a booster dose of BCG did not improve protection aginst TB [15].
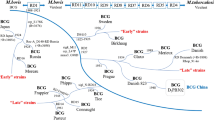
BCG is a safe vaccine in healthy infants. Loco-regional adverse reaction, including regional adenitis are usually self-limiting [12]. However, it can cause severe disseminated disease, named BCG-osis, in patients affected by some primary immunodeficiency, such as severe combined immunodeficiency and chronic granulomatous disease [16]. Different studies revealed an increased risk of disseminated BCG disease in HIV-infected children, even if asymptomatic at time of vaccination [17, 18]. Thus, WHO Global Advisory Committee on Vaccine Safety recommends that BGC should not be administered in HIV-infected patients [19]. This is an important limitation, considering that countries where TB is endemic are the same where HIV spreads. Since that, a safer and more effective vaccine is urgently required. In response to this challenge, the development pipeline now includes 12 vaccine candidates [20], that can either boost BCG’s initial effects (booster vaccine) or replace BCG (priming vaccine).
The aim of booster vaccines, that include viral vectored or protein adjuvant vaccines, is to enhance the immune response of BCG, whereas the priming vaccines aim at replacing BCG with a safer and long-lasting vaccine, that can be a recombinant BCG strain or a genetically attenuated Mtb.
A third strategy is to develop therapeutic vaccines reducing duration of TB therapy.
The aim of this review is to provide updated information on vaccine entered into clinical trials.
Methods
Data included in this review were retrieved by searches of Pubmed, references of the relevant articles and open-access website of Aeras and ClinicalTrial.gov. The search was limited to English-language studies published between 1st September, 2003 and 1st September 2013. Studies reporting data on vaccine now in clinical trials were included in this review. Article were excluded if redundant or not pertinent on the basis of titles and abstracts. Articles on pre-clinical studies of vaccine not yet in clinical trial were excluded.
Search strategy
The search strategy was: Vaccine[Title] AND (tuberculosis[Title] OR tb[Title] OR bcg[Title]) AND ("2003/09/01"[PDAT] : "2013/09/01"[PDAT]) AND (Journal Article[ptyp] AND English[lang]) NOT ("leishmania"[MeSH Terms] OR "leishmania"[All Fields]) NOT ("leprosy"[MeSH Terms] OR "leprosy"[All Fields]) NOT ("neoplasms"[MeSH Terms] OR "neoplasms"[All Fields] OR "cancer"[All Fields]) NOT ("hypersensitivity"[MeSH Terms] OR "hypersensitivity"[All Fields] OR "allergy"[All Fields] OR "allergy and immunology"[MeSH Terms] OR ("allergy"[All Fields] AND "immunology"[All Fields]) OR "allergy and immunology"[All Fields]) NOT ("vitamins"[Pharmacological Action] OR "vitamins"[MeSH Terms] OR "vitamins"[All Fields] OR "vitamin"[All Fields]) NOT ("lactoferrin"[MeSH Terms] OR "lactoferrin"[All Fields]).
Results
The search performed generated a total of 461 results. A total of 100 articles were included in the review (Additional file 1). Three viral vectored booster vaccines, five protein adjuvant booster vaccines, two priming vaccines and two therapeutic vaccines have been analyzed.
Viral-vectored booster vaccines
MVA85A
MVA85A is the most clinically advanced vaccine candidate (Table 1). It is the first new TB vaccine to enter into clinical trials in 2002 and to be tested in infants since BCG.
It is a recombinant strain of Modified Vaccinia virus Ankara expressing the Mtb antigen 85A (Ag85A), designed to enhance response induced by BCG [21]. The live viral vector cannot replicate in human.
Several trials reported its safety in healthy adults, Mtb and HIV infected patients, adolescents, children and infants[22–26]. Minor local and systemic reactions are frequently reported in the first week after immunization. No serious vaccine-related adverse events have been reported [22–26].
Vaccine is normally performed intradermally, even if a phase I trial reported a safe and higher immunogenic profile of MVA85A delivered intramuscularly in healthy adults [27].
MVA85A has shown to induce a polyfunctional CD4+ T-cells population, expressing INF-γ, interleukin-2 (IL-2), tumour necrosis factor-α (TNF-α), interleukin-17 (IL-17) and granulocyte-macrophage colony-stimulating factor and a modest CD8+ T-cells response [22, 25, 26, 28, 29].
Data showed that a dose of 1 x 108 plaque forming units was as safe as lower doses and induced a higher immune response in healthy previously BCG vaccinated UK adults [23].
However, a recent phase 2b trial on safety and efficacy of MVA85A was conducted in 2797 healthy South African infants previously vaccinated with BCG. The vaccine was well tolerated and immunogenic, but it was poorly protective against TB infection [22]. Authors suggested that the immunologic response induced by the vaccine could not be related to protective effect against TB infection.
The safety and immunogenicity of MVA85A priming and BCG-booster vaccination is currently being evaluated in a phase 2 trial [30].
Moreover, a phase 1 trial testing safety of MVA85A combined with a different carrier protein, IMX313, is ongoing [31].
Another poxvirus-vectored candidate vaccine, Fowlpox virus expressing the Mtb antigen 85A has been entered clinical trial in 2008 but it failed to induce an adequate immune-response [32].
AdAg85a
AdAg85a is a recombinant strain of replication-deficient adenoviral vector expressing the Mtb Ag85A [33] (Table 1).
Experimental data showed that it provided potent protection against pulmonary TB infection in mice when administered intranasally, either as priming and as booster vaccine for BCG [33, 34]. Intranasal administration enhanced a better protection than intramuscular in both settings [33, 34]. In guinea pigs, both intranasal and intramuscular vaccination were protective against pulmonary TB infection. However intranasal route seemed to provide stronger protection [35].
Santuosso et al. demonstrated that intranasal AdAg85a was able to elicit robust mucosal CD4+ and CD8+ T-cells responses in the airway lumen [34, 36].
A phase 1 trial evaluating safety and immunogenicity of AdAg85a administered intramuscularly in previously and not-previously BCG-vaccinated healthy adults has been terminated in July 2013 [37].
Mu et al. reported that a new intranasally bivalent adenovirus-vectored vaccine expressing Ag85A and TB10.4 antigen provided an improved protection against TB pulmonary infection in mice [38].
Ad35/Aeras-402
Ad35/Aeras 402 is a recombinant, non-replicating adenovirus, serotype 35 vaccine, which expresses a fusion protein from the Mtb Ag85A, antigens 85B (Ag85B) and TB10.4 [39] (Table 1).
Good safety profiles have been shown in phase 1 trials on healthy previously BCG vaccinated adults. No serious adverse events related to the vaccine have been reported. However, mild to moderate local adverse events were frequent [40, 41].
Ad35/Areas 402 provided strong CD4+ and CD8+ T-cells responses in mouse, especially if intranasally administered [39]. Similarly, it was able to induce potent CD4+ and CD8+ T-cells responses in healthy adults, with important IFN-γ, TNF-α and IL-2 production if administered as booster BCG vaccine [40, 41]. However, it did not elicit any IL-17 secretion [41].
Trials evaluating safety and immunogenicity of Ad35/Aeras 402 in infants and HIV-infected adults are ongoing [42, 43]. Moreover, a study to asses safety and immunogenicity of Ad35/Aeras 402 followed by MVA85A started on September 2012 [44].
Protein adjuvant booster vaccines
H1/IC31
H1/IC31 is a recombinant subunit vaccine, composed by the hybrid protein of Early Secretory Antigenic Target 6 (ESAT6) and Ag85B adjuvanted with IC31, an adjuvant system composed by the cationic protein polyaminoacid KLK and oligodeoxynucleotide ODN1a [45] (Table 2).
The fusion protein Ag85B-ESAT6 has been extensively evaluated in several animal models and combined with different adjuvants [46–49].
H1/IC31 has been shown to be safe in healthy adults. No serious adverse events related to the vaccine have been reported. Local and systemic described events were mild and quickly resolving (<48 hours) [45, 50, 51].
H1/IC31 provided long-lasting TH1 responses either in mycobacterially-naïve subjects as well in BCG-vaccinated or previously TB infected voluntaries. The immune response can be amplified by booster vaccinations. H1/IC31 induce a weak immune response against ESAT6; however this vaccine provided a more potent protective effect than a vaccine with Ag85B alone [45, 50, 51].
IGRAs measure the amount of INF-γ produced by lymphocytes after in vitro incubation with Mtb antigens, included ESAT6. Even if H1/IC31 has shown to induce QuantiFERON positivity in only few subjects, further studies evaluating possible interference of the vaccine with IGRA results are needed [45, 50, 51].
HyVac4/Aeras 404
HyVac4/Aeras 404 is a booster vaccine developed by the same group of H1/IC31. The antigen ESAT6 was replaced by TB10.4, to avoid the interference with IGRAs and the fusion protein was combined with the adjuvant IC31 [52] (Table 2).
Dietrich et al. demonstrated that the fusion protein Ag85B-TB10.4 (HyVac4) combined with the adjuvant protein dimethyl dioctadecyl ammonium/monophosphoryl lipid A (DDA/MPL) was highly immunogenic and induced strong protection against TB in mice model [53].
HyVac 4/Aeras 404 is safe and protective against pulmonary TB when administered as priming or booster vaccine in guinea pigs and mice [52, 54]. When used as booster vaccine for BCG in mouse model, it induced expression of IFN-γ, TNF-α and IL-2 triple positive CD4+ T-cells that seemed to be correlated with protection against TB [54].
Several phase I trials on HyVac4/Aeras 404 have been terminated [55] and a phase I/II trial on its safety and immunogenicity in BCG vaccinated healthy infants is ongoing [56].
ID93/GLA-SE
ID93/GLA-SE is a protein-adjuvant vaccine, composed by ID93, a fusion protein comprising four Mtb antigens (Rv2608, Rv3619, Rv3620 and Rv1813), combined with the glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE) [57] (Table 2).
ID93/GLA-SE induced a significant TH1 immune response, with multifunctional IFN-γ, TNF-α and IL-2 CD4+ T-cells production in BCG-vaccinated or not-vaccinated mice and guinea pigs [57, 58]. That response led a strong protection against pulmonary TB. Moreover, it was well tolerated and induced TH1 and TH2 CD4+T-cells responses in BCG-vaccinated non-humans primates and it elicited CD4+ and CD8+ T-cells responses in BCG-vaccinated or TB-exposed human peripheral blood mononuclear cells [57]. In contrast, if combined with SE alone, ID93 elicited a Th2 immune response, that did not improve protection against pulmonary TB in mouse and guinea pig models [58].
Experimental data showed that ID93/GLA-SE protected also against MDR-TB in animal models [57].
Baldwin et al. demonstrated that ID93 combined with an adenovirus type 5 vector induced strong CD8+ T-cells responses, but it did not provide long-lived immune responses if not combined with a priming or booster ID93/GLA-SE vaccination [59]
Two clinical trials evaluating safety, tolerability and immunogenicity of ID93/GLA-SE in healthy adults, either as priming vaccine as well as booster vaccine, are ongoing [60, 61].
H56/IC31
H56/IC31 is a protein-adjuvant vaccine composed by H56, a fusion protein containing Ag85B, ESAT6 and the latency-associated protein Rv2660c, combined with the adjuvant IC31 [62] (Table 2). In mouse models, H56 elicited a strong multifunctional CD4+T-cells response and limited reactivation of latent TB [63].
Lin et al. demonstrated that H56/IC31 was safe and immunogenic in BCG-vaccinated non-human primate models. Moreover the vaccine showed excellent control of latent infection [62]. Notably, in vaccinated non-human primate anti-TNF antibodies treatment did not induce reactivation of latent TB [62].
A phase 1/2a trial on safety and immunogenicity of H56/IC31 in HIV-negative, BCG vaccinated with or without latent TB is ongoing [64].
M72/AS01E
M72/AS01E is a recombinant vaccine developed to boost BCG-induced or Mtb-induced immune response (Table 2).
The M72 antigen is strictly related to Mtb72F, a fusion protein comprising the Mtb39a and Mtb32a antigens [65]. A point mutation was performed in the Mtb32a antigen of M72 to improve the long-term stability of Mtb72F [66].
AS01E is an adjuvant system containing the immunostimulants MPL and Quillaja saponaria fraction 1 (QS21) combined with liposomes [66]. It induced humoral and TH1 cellular responses [65].
Mtb72F, combined with the adjuvant AS02 (oil-in-water emulsion of MPL and QS21) has been tested in mice, guinea pigs, rabbits and non-human primates models, revealed good safety and immunogenicity profiles [67–69]. Moreover, Mtb72F/AS02 was rather well tolerated and immunogenic in adults with or without previous exposure to Mtb or BCG [65, 70].
Meanwhile, concerns for the real effectiveness of Mtb72f have emerged do to variation in Mtb32a and Mtb39a antigens sequences in different Mtb strains [71].
M72/AS01E presented frequent local adverse events, resolving within one week of vaccination, predominantly due to the adjuvant. No serious adverse events related to the vaccination have been reported [66, 72].
M72/AS01E induced long-lasting multifunctional CD4+T-cells responses in adults with or without BCG or Mtb contacts, that was higher than the responses elicited by the AS02-adjuvanted vaccine. As shown in MVA85A, M72/AS01E seemed not to induce robust CD8+T-cells responses [66, 72].
Phase 2 trials on safety and immunogenicity of M72/AS01E in adults with HIV and with TB are ongoing [73, 74].
Priming vaccines
VPM1002
VPM1002 is a recombinant BCG strain that expresses membrane-perforating listeriolysin (encoded by the gene hly) of Listeria monocytogenes, lacking the urease C gene (BCG ΔureC::hly) and that contains a hygromycin resistance marker [75] (Table 3).
It has shown to produce a better protection against TB by stimulating type 1 and type 17 cytokines in mice compared with parental BCG (pBCG) [76]. Moreover, in a mouse model, apoptotic vescicles from BCG ΔureC::hly-infected macrophages induced greater CD4+ and CD8+ T-cells responses than pBCG [77].
VPM1002 was safe in healthy adults, with adverse events profile comparable to BCG. No serious adverse events related to the vaccine have been reported and no human-to-human transmission has been documented [75]. VPM1002 induced robust CD4+ and CD8+ T-cells and antibodies responses [75].
A phase 2 trial evaluating safety and immunogenicity of VPM1002 in comparison with BCG in newborns is ongoing [78].
Notably, the recombinant BCG strain Aeras 422, that showed promising results in animal models , failed to overcome phase 1 trial, do to some reactivation of shingles in vaccinated healthy adults [79] and the development of rBCG30 strain, an overexpressing Ag85B recombinant vaccine that showed safety and immunogenicity in a phase 1 trial, did not carry on [80].
MTBVAC
MTBVAC is the first live-attenuated Mtb vaccine entered in phase 1 clinical trial in January 2013 [81] (Table 3).
It derives from the SO2, an attenuated strain obtained by the insertion of a kanamycin-resistance cassette in the phoP gene of Mtb. phoP is a transcription regulator, therefore its mutation determines lack of expression of several genes, including virulence factors, such as ESAT6 [82]. Although SO2 seemed to be immunogenic and protective against TB in animal models [82, 83], it failed to satisfy the Geneva consensus requirements for progressing new vaccines into clinical trials [81].
Hence, the same research group developed a new vaccine strain, with tow stable mutations in the phoP and fadD26 genes [81]. fadD26 product is required for the synthesis of phthiocerol dimycocerosates, a component of cell envelope that protect Mtb from host defenses [84].
MTBVAC was safe, immunogenic and protective against TB in mouse and guinea pig models [81]. Since it was functionally comparable to SO2, it has been authorized to enter in clinical trial after these tests.
Therapeutic vaccines
RUTI®
RUTI® is a therapeutic vaccine constituted by detoxified liposomal fragments of Mtb [85] (Table 4). It was developed to complete latent TB treatment after a short course of antimicrobial therapy [85, 86]. Experimental data showed that RUTI® is safe and able to elicit TH1-TH2-TH3 responses in animal models. Moreover, CD8+ T-cells and antibodies responses have been reported [85, 87].
A phase 1 trial revealed that RUTI® administered subcutaneously was rather well tolerated in BCG-naïve healthy adults, except local reaction. No serious adverse events have been reported [88]. Moreover, it induced a specific cellular and humoral responses [88].
Mycobacterium vaccae
A whole inactivated Mycobacterium vaccae (MV) administered intradermally was firstly evaluated as a therapeutic vaccine (Table 4). Clinical trials showed conflicting results [89]. A meta-analysis on MV added TB chemotherapy in never-treated TB patients, showed that it is effective in improving both sputum conversion and X-ray imagines [90].
More recently, MV was tested as prophylactic vaccine to prevent TB, especially in HIV-infected patients [91–93]. MV was well tolerated and no serious adverse events have been reported. A meta-analysis revealed that MV was able to prevent TB in high risk category and was safe and immunogenic in HIV-infected patients [94].
In a phase 3 trial, MV has shown to induce variable IFN-γ and humoral responses, according to CD4+ T-cells count, HIV viral load and previous TB treatment [95].
Conclusions
One of the WHO’s MDG is to reduce TB incidence by 2015 and one of the Stop TB Partnership targets is to eradicate TB by 2050. Hence, combined strategies based on faster diagnostic tools, drugs effective against MDR TB and able to shortness duration of treatment and vaccines are essential to reach these targets.
Several vaccines against TB are in the pipeline, either as priming, booster and therapeutic vaccines. Since the three options operate at different levels (pre-exposure or post-exposure), they can be considered complementary and hopefully they can succeed in eradicating TB.
Notably, as seen for MVA85A, vaccines that resulted to be immunogenic in animal models and humans can failed to show effectiveness in late phase trials [22]. Therefore, immune mechanisms of protection against TB should be simultaneously explored.
The existence of several lines of research can mean that a main road does not exist at the present.
Finally, even if several vaccines are in the pipeline, further investments on basic research, clinical testing and mass vaccination campaigns are essential to achieve the ambitious goals of eradication.
Abbreviations
- WHO:
-
World Health Organization
- MDG:
-
Millennium Development Goal
- TB:
-
tuberculosis
- HIV:
-
human immunodeficiency virus
- MDR:
-
multidrug-resistant
- BCG:
-
bacille Calmette-Guérin
- IGRA:
-
interferon-γ release assay
- Ag85A:
-
antigen 85A
- IL-2:
-
interleukin-2
- TNF-α:
-
tumour necrosis factor-α
- IL-17:
-
interleukin-17
- Ag85B:
-
antigen 85B
- ESAT6:
-
Early Secretory Antigenic Target 6
- DDA/MPL:
-
dimethyl dioctadecyl ammonium/monophosphoryl lipid A
- GLA/SE:
-
glucopyranosyl lipid adjuvant-stable emulsion
- QS21:
-
Quillaja saponaria fraction 1
- pBCG:
-
parental BCG
- MV:
-
Mycobacterium vaccae
References
Raviglione MC, Uplekar MW: WHO's new Stop TB Strategy. Lancet. 2006, 367: 952-955. 10.1016/S0140-6736(06)68392-X.
World Health Organization: Global tuberculosis report 2012. [http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf]
Ottenhoff TH, Kaufmann SH: Vaccines against tuberculosis: where are we and where do we need to go?. PLoS Pathog. 2012, 8: e1002607-10.1371/journal.ppat.1002607.
Beresford B, Sadoff JC: Update on research and development pipeline: tuberculosis vaccines. Clin Infect Dis. 2010, 50 (Suppl 3): 178-183.
Liu J, Tran V, Leung AS, Alexander DC, Zhu B: BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin. 2009, 5: 70-78. 10.4161/hv.5.2.7210.
Behr MA: BCG--different strains, different vaccines?. Lancet Infect Dis. 2002, 2: 86-92. 10.1016/S1473-3099(02)00182-2.
Hesseling AC, Cotton MF, Fordham von Reyn C, Graham SM, Gie RP, Hussey GD: Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. Int J Tuberc Lung Dis. 2008, 12: 1376-1379.
Dalmia N, Ramsay AJ: Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines. 2012, 11: 1221-33. 10.1586/erv.12.94.
Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV: The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995, 96: 29-35.
Trunz BB, Fine P, Dye C: Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006, 367: 1173-1180. 10.1016/S0140-6736(06)68507-3.
Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F: Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994, 271: 698-702. 10.1001/jama.1994.03510330076038.
World Health Organization: Issues relating to the use of BCG in immunization programmes. A discussion document. [http://www.who.int/vaccine_safety/committee/topics/bcg/en/]
Fine PE: Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995, 346: 1339-45. 10.1016/S0140-6736(95)92348-9.
Basu Roy R, Sotgiu G, Altet-Gómez N, Tsolia M, Ruga E, Velizarova S, Kampmann B: Identifying predictors of interferon-γ release assay results in pediatric latent tuberculosis: a protective role of bacillus Calmette-Guerin?: a pTB-NET collaborative study. Am J Respir Crit Care Med. 2012, 186: 378-384. 10.1164/rccm.201201-0026OC.
Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, Hijjar MA, Dourado I, Cruz AA, Sant'Anna C, Bierrenbach AL, Barreto ML: Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005, 366: 1290-1295. 10.1016/S0140-6736(05)67145-0.
Norouzi S, Aghamohammadi A, Mamishi S, Rosenzweig SD, Rezaei N: Bacillus Calmette-Guérin (BCG) complications associated with primary immunodeficiency diseases. J Infect. 2012, 64: 543-554. 10.1016/j.jinf.2012.03.012.
Fallo A, Torrado L, Sanchez A, Cerqueiro C, Shadgrosky L, Lopez EL: Delayed complications of Bacillus Calmette- Guerin (BCG) vaccination in HIV infected children [abstract WeOa0104]. The 3rd IAS Conference on HIV Pathogenesis and Treatment: 24-27. 2005, [http://iset.aids2010.org/Abstracts/A2176496.aspx]July ; Rio de Janeiro
Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N: The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007, 25: 14-18. 10.1016/j.vaccine.2006.07.020.
Global Advisory Committee on Vaccine Safety, 29-30 November 2006. Wkly Epidemiol Rec. 2007, 82: 18-24.
Stop TB partnership: Tuberculosis vaccine in clinical development. [http://www.stoptb.org/wg/new_vaccines/documents.asp]
McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV: Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis. 2005, 85: 47-52. 10.1016/j.tube.2004.09.015.
Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85A 020 Trial Study Team: Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013, 381: 1021-1028. 10.1016/S0140-6736(13)60177-4.
Pathan AA, Minassian AM, Sander CR, Rowland R, Porter DW, Poulton ID, Hill AV, Fletcher HA, McShane H: Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine. 2012, 30: 5616-5624. 10.1016/j.vaccine.2012.06.084.
Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, Mauff K, Moyo S, Brittain N, Lawrie A, Mulenga H, de Kock M, Makhethe L, Janse van Rensburg E, Gelderbloem S, Veldsman A, Hatherill M, Geldenhuys H, Hill AV, Hawkridge A, Hussey GD, Hanekom WA, McShane H, Mahomed H: A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012, 185: 769-778. 10.1164/rccm.201108-1548OC.
Minassian AM, Rowland R, Beveridge NE, Poulton ID, Satti I, Harris S, Poyntz H, Hamill M, Griffiths K, Sander CR, Ambrozak DR, Price DA, Hill BJ, Casazza JP, Douek DC, Koup RA, Roederer M, Winston A, Ross J, Sherrard J, Rooney G, Williams N, Lawrie AM, Fletcher HA, Pathan AA, McShane H: A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open. 2011, 1: e000223-
Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Isaacs F, Keyser A, Moyo S, Brittain N, Lawrie A, Gelderbloem S, Veldsman A, Hatherill M, Hawkridge A, Hill AV, Hussey GD, Mahomed H, McShane H, Hanekom WA: Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010, 40: 279-290.
Meyer J, Harris SA, Satti I, Poulton ID, Poyntz HC, Tanner R, Rowland R, Griffiths KL, Fletcher HA, McShane H: Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine. 2013, 31: 1026-1033. 10.1016/j.vaccine.2012.12.042.
Griffiths KL, Pathan AA, Minassian AM, Sander CR, Beveridge NE, Hill AV, Fletcher HA, McShane H: Th1/Th17 cell induction and corresponding reduction in ATP consumption following vaccination with the novel Mycobacterium tuberculosis vaccine MVA85A. PLoS One. 2011, 6: e23463-10.1371/journal.pone.0023463.
Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, Mulenga H, de Kock M, Gelderbloem S, Veldsman A, Hatherill M, Geldenhuys H, Hill AV, Hussey GD, Mahomed H, Hanekom WA, McShane H: Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis. 2011, 203: 1832-1843. 10.1093/infdis/jir195.
Safety and immunogenicity of MVA85A prime and Bacille Calmette-Guerin boost vaccination. [http://clinicaltrials.gov/ct2/show/NCT01650389?term=NCT01650389&rank=1]
Phase I trial evaluating safety and immunogenicity of MVA85A-IMX313 compared to MVA85A in BCG vaccinated adults. [http://clinicaltrials.gov/ct2/show/NCT01879163?term=mva85a&recr=Open&rank=1]
Rowland R, Pathan AA, Satti I, Poulton ID, Matsumiya MM, Whittaker M, Minassian AM, O'Hara GA, Hamill M, Scott JT, Harris SA, Poyntz HC, Bateman C, Meyer J, Williams N, Gilbert SC, Lawrie AM, Hill AV, McShane H: Safety and immunogenicity of an FP9-vectored candidate tuberculosis vaccine (FP85A), alone and with candidate vaccine MVA85A in BCG-vaccinated healthy adults: a phase I clinical trial. Hum Vaccin Immunother. 2013, 9: 50-62. 10.4161/hv.22464.
Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z: Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004, 173: 6357-6365. 10.4049/jimmunol.173.10.6357.
Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z: Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun. 2006, 74: 4634-4643. 10.1128/IAI.00517-06.
Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN: Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One. 2009, 4: e5856-10.1371/journal.pone.0005856.
Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z: Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005, 174: 7986-7994. 10.4049/jimmunol.174.12.7986.
Study of the Safety and Immunogenicity of an Adenovirus-based Tuberculosis Vaccine. [http://clinicaltrials.gov/ct2/results?term=nct00800670&Search=Search]
Mu J, Jeyanathan M, Small CL, Zhang X, Roediger E, Feng X, Chong D, Gauldie J, Xing Z: Immunization with a bivalent adenovirus-vectored tuberculosis vaccine provides markedly improved protection over its monovalent counterpart against pulmonary tuberculosis. Mol Ther. 2009, 17: 1093-1100. 10.1038/mt.2009.60.
Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, van der Poll T, Havenga M, Goudsmit J: Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun. 2007, 75: 4105-4115. 10.1128/IAI.00004-07.
Hoft DF, Blazevic A, Stanley J, Landry B, Sizemore D, Kpamegan E, Gearhart J, Scott A, Kik S, Pau MG, Goudsmit J, McClain JB, Sadoff J: A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine. 2012, 30: 2098-2108. 10.1016/j.vaccine.2012.01.048.
Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA: The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010, 181: 1407-1417. 10.1164/rccm.200910-1484OC.
Study of AERAS-402 in Healthy Infants. [http://clinicaltrials.gov/ct2/show/NCT01198366?term=aeras+402&rank=4]
Safety and Immunogenicity of AERAS-402 in HIV-infected, Bacillus Calmette-Guerin (BCG)-Vaccinated Adults. [http://clinicaltrials.gov/ct2/show/NCT01017536?term=aeras+402&rank=2]
Safety Study of Tuberculosis Vaccines AERAS-402 and MVA85A. [http://clinicaltrials.gov/ct2/show/NCT01683773?term=aeras+402&rank=1]
van Dissel JT, Soonawala D, Joosten SA, Prins C, Arend SM, Bang P, Tingskov PN, Lingnau K, Nouta J, Hoff ST, Rosenkrands I, Kromann I, Ottenhoff TH, Doherty TM, Andersen P: Ag85B-ESAT-6 adjuvanted with IC31® promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in volunteers with previous BCG vaccination or tuberculosis infection. Vaccine. 2011, 29: 2100-2109. 10.1016/j.vaccine.2010.12.135.
Ingvarsson PT, Schmidt ST, Christensen D, Larsen NB, Hinrichs WL, Andersen P, Rantanen J, Nielsen HM, Yang M, Foged C: Designing CAF-adjuvanted dry powder vaccines: spray drying preserves the adjuvant activity of CAF01. J Control Release. 2013, 167: 256-264. 10.1016/j.jconrel.2013.01.031.
You Q, Wu Y, Wu Y, Wei W, Wang C, Jiang D, Yu X, Zhang X, Wang Y, Tang Z, Jiang C, Kong W: Immunogenicity and protective efficacy of heterologous prime-boost regimens with mycobacterial vaccines and recombinant adenovirus- and poxvirus-vectored vaccines against murine tuberculosis. Int J Infect Dis. 2012, 16: e816-825. 10.1016/j.ijid.2012.07.008.
Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R, Agger EM, Aagaard C, Weiler H, van Soolingen D, Dalemans W, Thomas AW, Andersen P: Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005, 23: 2740-2750. 10.1016/j.vaccine.2004.11.051.
Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P: Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004, 72: 6148-6150. 10.1128/IAI.72.10.6148-6150.2004.
van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff TH, Kromann I, Doherty TM, Andersen P: Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naïve human volunteers. Vaccine. 2010, 28: 3571-3581. 10.1016/j.vaccine.2010.02.094.
Ottenhoff TH, Doherty TM, van Dissel JT, Bang P, Lingnau K, Kromann I, Andersen P: First in humans: a new molecularly defined vaccine shows excellent safety and strong induction of long-lived Mycobacterium tuberculosis-specific Th1-cell like responses. Hum Vaccin. 2010, 6: 1007-1015. 10.4161/hv.6.12.13143.
Skeiky YA, Dietrich J, Lasco TM, Stagliano K, Dheenadhayalan V, Goetz MA, Cantarero L, Basaraba RJ, Bang P, Kromann I, McMclain JB, Sadoff JC, Andersen P: Non-clinical efficacy and safety of HyVac4:IC31 vaccine administered in a BCG prime-boost regimen. Vaccine. 2010, 28: 1084-1093. 10.1016/j.vaccine.2009.10.114.
Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P: Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005, 174: 6332-6339. 10.4049/jimmunol.174.10.6332.
Billeskov R, Elvang TT, Andersen PL, Dietrich J: The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One. 2012, 7: e39909-10.1371/journal.pone.0039909.
AERAS-404/HyVac4. [http://192.240.162.26/candidates]
Phase 1/II, Safety and Immunogenicity Study of AERAS-404 in BCG-Primed Infants. [http://clinicaltrials.gov/ct2/results?term=aeras+404&Search=Search]
Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG: A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010, 2: 53-74.
Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN: The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012, 188: 2189-2197. 10.4049/jimmunol.1102696.
Baldwin SL, Ching LK, Pine SO, Moutaftsi M, Lucas E, Vallur A, Orr MT, Bertholet S, Reed SG, Coler RN: Protection against Tuberculosis with Homologous or Heterologous Protein/Vector Vaccine Approaches Is Not Dependent on CD8+ T Cells. J Immunol. 2013, 191: 2514-2525. 10.4049/jimmunol.1301161.
Phase 1 ID93 + GLA-SE Vaccine Trial in Healthy Adult Volunteers. [http://clinicaltrials.gov/ct2/show/NCT01599897?term=id93+gla&rank=1]
Phase 1 ID93 + GLA-SE Vaccine Trial in BCG-Vaccinated Healthy Adult Volunteers. [http://clinicaltrials.gov/ct2/show/NCT01927159?term=id93+gla&rank=2]
Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P: The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. 2012, 122: 303-314. 10.1172/JCI46252.
Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P: A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011, 17: 189-194. 10.1038/nm.2285.
A Phase I/IIa Safety & Immunogenicity of AERAS-456 in HIV-Negative Adults With & Without Latent Tuberculosis Infection. [http://clinicaltrials.gov/ct2/show/NCT01865487?term=h56+ic31&rank=1]
Von Eschen K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, Koutsoukos M, Moris P, Cain D, Dubois MC, Cohen J, Ballou WR: The candidate tuberculosis vaccine Mtb72F/AS02A: Tolerability and immunogenicity in humans. Hum Vaccin. 2009, 5: 475-482. 10.4161/hv.8570.
Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitié MA, Mettens P, Moris P, Ledent E, Leroux-Roels G, Ofori-Anyinam O, M72 Study Group: Improved CD4+ T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013, 31: 2196-2206. 10.1016/j.vaccine.2012.05.035.
Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y: Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A. 2009, 106: 2301-2306. 10.1073/pnas.0712077106.
Tsenova L, Harbacheuski R, Moreira AL, Ellison E, Dalemans W, Alderson MR, Mathema B, Reed SG, Skeiky YA, Kaplan G: Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006, 74: 2392-2401. 10.1128/IAI.74.4.2392-2401.2006.
Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, Basaraba RJ, Izzo AA, Lasco TM, Chapman PL, Reed SG, Orme IM: The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M.tuberculosis-infected guinea pigs. Infect Immun. 2004, 72: 6622-6632. 10.1128/IAI.72.11.6622-6632.2004.
Spertini F, Audran R, Lurati F, Ofori-Anyinam O, Zysset F, Vandepapelière P, Moris P, Demoitié MA, Mettens P, Vinals C, Vastiau I, Jongert E, Cohen J, Ballou WR: The candidate tuberculosis vaccine Mtb72F/AS02 in PPD positive adults: a randomized controlled phase I/II study. Tuberculosis. 2013, 93: 179-188. 10.1016/j.tube.2012.10.011.
McNamara LA, He Y, Yang Z: Using epitope predictions to evaluate efficacy and population coverage of the Mtb72f vaccine for tuberculosis. BMC Immunol. 2010, 11: 18-10.1186/1471-2172-11-18.
Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, Erasmus M, Makhethe L, Hughes EJ, Gelderbloem S, Bollaerts A, Bourguignon P, Cohen J, Demoitié MA, Mettens P, Moris P, Sadoff JC, Hawkridge A, Hussey GD, Mahomed H, Ofori-Anyinam O, Hanekom WA: Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med. 2013, 188: 492-502. 10.1164/rccm.201208-1385OC.
Safety and Immunogenicity of a Candidate Tuberculosis (TB) Vaccine in Adults With TB Disease. [http://clinicaltrials.gov/ct2/show/NCT01424501?term=692342&recr=Open&rank=1]
Study to Evaluate the Efficacy of GlaxoSmithKline (GSK) Biologicals' Candidate Tuberculosis (TB) Vaccine in Healthy Adults. [http://clinicaltrials.gov/ct2/show/NCT01755598?term=692342&recr=Open&rank=2]
Grode L, Ganoza CA, Brohm C, Weiner J, Eisele B, Kaufmann SH: Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013, 31: 1340-1348. 10.1016/j.vaccine.2012.12.053.
Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH: Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis. 2011, 204: 1573-1584. 10.1093/infdis/jir592.
Farinacci M, Weber S, Kaufmann SH: The recombinant tuberculosis vaccine rBCG ΔureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells. Vaccine. 2012, 30: 7608-7614. 10.1016/j.vaccine.2012.10.031.
Study to Evaluate Safety and Immunogenicity of VPM1002 in Comparison With BCG in Newborn Infants in South Africa. [http://clinicaltrials.gov/ct2/show/NCT01479972?term=vpm1002&rank=2]
Kaufmann SH, Gengenbacher M: Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012, 23: 900-907. 10.1016/j.copbio.2012.03.007.
Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA: A new recombinant bacille Calmette-Guérin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008, 198: 1491-1501. 10.1086/592450.
Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, Fernandez C, Parra A, Cardona PJ, Vilaplana C, Ausina V, Williams A, Clark S, Malaga W, Guilhot C, Gicquel B, Martin C: Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013, 31: 4867-4873. 10.1016/j.vaccine.2013.07.051.
Nambiar JK, Pinto R, Aguilo JI, Takatsu K, Martin C, Britton WJ, Triccas JA: Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. Eur J Immunol. 2012, 42: 385-392. 10.1002/eji.201141903.
Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, Soto CY, Clark SO, Hatch GJ, Aguilar D, Ausina V, Gicquel B: The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006, 24: 3408-3419. 10.1016/j.vaccine.2006.03.017.
Kaufmann SH, Gengenbacher M: Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012, 23: 900-7. 10.1016/j.copbio.2012.03.007.
Cardona PJ: RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis. 2006, 86: 273-289. 10.1016/j.tube.2006.01.024.
Vilaplana C, Gil O, Cáceres N, Pinto S, Díaz J, Cardona PJ: Prophylactic effect of a therapeutic vaccine against TB based on fragments of Mycobacterium tuberculosis. PLoS One. 2011, 6: e20404-10.1371/journal.pone.0020404.
Vilaplana C, Ruiz-Manzano J, Gil O, Cuchillo F, Montané E, Singh M, Spallek R, Ausina V, Cardona PJ: The tuberculin skin test increases the responses measured by T cell interferon-gamma release assays. Scand J Immunol. 2008, 67: 610-617. 10.1111/j.1365-3083.2008.02103.x.
Vilaplana C, Montané E, Pinto S, Barriocanal AM, Domenech G, Torres F, Cardona PJ, Costa J: Double-blind, randomized, placebo-controlled Phase I Clinical Trial of the therapeutical antituberculous vaccine RUTI. Vaccine. 2010, 28: 1106-1116. 10.1016/j.vaccine.2009.09.134.
Dlugovitzky D, Fiorenza G, Farroni M, Bogue C, Stanford C, Stanford J: Immunological consequences of three doses of heat-killed Mycobacterium vaccae in the immunotherapy of tuberculosis. Respir Med. 2006, 100: 1079-1087. 10.1016/j.rmed.2005.09.026.
Yang XY, Chen QF, Li YP, Wu SM: Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS One. 2011, 6: e23826-10.1371/journal.pone.0023826.
Lahey T, Arbeit RD, Bakari M, Horsburgh CR, Matee M, Waddell R, Mtei L, Vuola JM, Pallangyo K, von Reyn CF: Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine. 2010, 28: 7652-7658. 10.1016/j.vaccine.2010.09.041.
von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, Matee M, Bakari M, Tvaroha S, Adams LV, Horsburgh CR, Pallangyo K, DarDar Study Group: Prevention of tuberculosis in Bacille Calmette-Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010, 24: 675-685. 10.1097/QAD.0b013e3283350f1b.
Vuola JM, Ristola MA, Cole B, Järviluoma A, Tvaroha S, Rönkkö T, Rautio O, Arbeit RD, von Reyn CF: Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: a randomized, controlled trial. AIDS. 2003, 17: 2351-2355. 10.1097/00002030-200311070-00010.
Yang XY, Chen QF, Cui XH, Yu Y, Li YP: Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: a meta-analysis. J Infect. 2010, 60: 320-330. 10.1016/j.jinf.2010.02.005.
Declarations
Publication charges were funded by the Italian Health Ministry and the Young Research Project. This article has been published as part of BMC Infectious Diseases Volume 14 Supplement 1, 2014: Highlights in Pediatric Tuberculosis. The full contents of the supplement are available online at http://www.biomedcentral.com/bmcinfectdis/supplements/14/S1
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that have no competing interests
Authors' contributions
CM, EC, LG conceived the idea, carried out the literature search, and drafted the manuscript. MdM contributed to devise and develop the idea and helped to draft and critically reviewed the manuscript.
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Montagnani, C., Chiappini, E., Galli, L. et al. Vaccine against tuberculosis: what’s new?. BMC Infect Dis 14 (Suppl 1), S2 (2014). https://doi.org/10.1186/1471-2334-14-S1-S2
Published:
DOI: https://doi.org/10.1186/1471-2334-14-S1-S2