Abstract
Background
The prevalence, genotypes, and vertical transmission characteristics of human papillomavirus (HPV) among pregnant women from Nanjing, China was investigated.
Methods
Cervical cells were collected from healthy pregnant women (n = 3139; stage of gestation, 24.6 ± 2.1 weeks) for cytological evaluation and determination of HPV infection status. Exfoliated oral and genital cells were collected from neonates (<1-day-old, n = 233) whose mothers were positive for HPV DNA. We used HPV Gene Chip technology with 23 HPV genotype probes to conduct our analysis.
Results
Overall prevalence of HPV DNA among pregnant women was 13.4% (422/3139). The most frequently detected HPV genotypes were HPV-16 (29.6%, 125/422), -18 (14.7%, 62/422), and -58 (14.2%, 60/422). The rate of concordance for HPV DNA in maternal-neonatal pairs was 23.6% (55/233), with HPV type-specific concordance occurring in 26 cases. A higher prevalence of HPV DNA was apparent in female neonates compared with males (17.7 vs. 11.6%).
Conclusions
The prevalence of cervical HPV DNA in pregnant women from Nanjing was low, with vertical transmission rates slightly higher. From our findings, we concluded that there was efficient vertical transmission of three HPV genotypes, with HPV-16 the most prevalent type in pregnant women and newborn babies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Globally, cervical cancer is the second-most commonly occurring cancer in women. In recent years, the occurrence of sexually transmitted infections (STIs) involving human papillomavirus (HPV) has been increasing in China, and are now second only to the incidence of STIs for gonorrhea [1]. HPV has been recognized as the primary cause of cervical cancer, papillomatosis, and anogenital warts [2, 3]. Low-risk, non-oncogenic forms of HPV are associated with anogenital warts and laryngeal papillomatosis; high-risk, oncogenic types are associated with cancers of the cervix, anogenital areas, head and neck [4].
In the early 1950s, a study revealed that pregnant women infected with HPV were able to vertically transmit the virus to neonates, resulting in infantile anal and genital condyloma acuminatum, congenital conjunctival papilloma, and juvenile laryngeal papillomatosis [5, 6]. A recent report involving 88 cases where the sexual life history of females was followed indicated that congenital infection of HPV might result in an increased risk of genital condyloma acuminatum [7]. However, geographic regions display different HPV genotype distribution among ethnicities. The current methods of virus detection are based upon rates of congenital HPV infection, which have been reported at 0–80.9% [8].
The five most prevalent HPV genotypes associated with genital and oral cancers in females are HPV-16, -18, -31, -58, and -52 [9]. Previous surveys in China have found HPV-52 and -58 to be more prevalent than HPV-16 in some areas [10, 11]; this distribution is consistent with those found in Japan and eastern Africa [9]. Most studies have found a higher prevalence of HPV DNA among pregnant women compared with those who are not pregnant [12–14].
It was previously shown, using DNA hybridization techniques, that HPV could not be detected in 11 oropharyngeal samples taken from neonates (1-day-old). However, from an age of 5 weeks up to 18 months, HPV DNA could be detected [15]. This suggests that the virus may be carried by the neonate even though it could not be detected by DNA hybridization. Mant et al. [16] reported using a more sensitive polymerase chain reaction (PCR) detection method to detect HPV-16 DNA in 20 neonatal oropharyngeal samples. After 30 months, eight samples were positive for virus.
Although there is overwhelming evidence for the sexual transmission of high-risk HPV genotypes, several studies have examined other routes, such as vertical transmission before or during childbirth, direct contact during labor, or horizontal transmission among children through contact with infected skin lesions [17, 18]. However, similar studies of transmission modes in different regions have obtained disparate results. Studies of vertical HPV transmission have reported a wide range of neonatal infection rates. These conflicting findings are primarily because of the differences in populations and experimental techniques, and might have been influenced by factors such as sex, type of delivery, and maternal status before delivery.
It is important to examine the distribution of HPV genotypes found in pregnant women, as it can guide the development of genotype vaccines for the prevention of cancer due to HPV infection [19]. The prevalence of vertical HPV transmission may have an important impact on clinical handling and vaccination strategies for infected women during pregnancy. We sought to determine the prevalence, types, and vertical transmission characteristics of HPV in pregnant women from Nanjing (Jiangsu Province, China).
Methods
Participants
Healthy pregnant women (n = 3139) were recruited, from a sample population of 11,696 women, who had their first obstetric examinations at the Affiliated Drum Tower Hospital (Nanjing University Medical School, China) between January 2006 and April 2010. A follow-up study was conducted after delivery. The exclusion criteria for this study were: (1) near miscarriage or abnormal vaginal bleeding; (2) cervical lesions apparent upon simple visual inspection during gynecological examination; (3) sexual intercourse and/or vaginal medication in the previous 3 days; (4) HPV detected by cervical cytological examination within 1 year; (5) mental or physical incompetence; (6) in vitro fertilization (IVF); and (7) refusal of gynecological examination. There were 1088 pregnant women that were excluded due to the exclusion criteria. The mean age of the 3139 participants was 29.9 years (range, 20–44 years). Most (81.5%, 2558/3139) women were in the 22nd–26th week of gestation upon enrollment in the study. All participating women provided written informed consent.
Data collection
Sociodemographic information for all participants, from January 2006 until April 2010, was obtained, along with gestational age when samples were collected, and parity data. A follow-up study was performed after delivery. Samples were collected from all pregnant women and from 233 infants of the 422 HPV-positive women due to some mother refusing examination and sampling of the infant. The sample collection was carefully executed to prevent cross-contamination between subjects and anatomical sites by using disposable equipment and changing the bed lining and collector’s gloves between each subject. Sample collection was carefully performed to prevent cross-contamination between subjects and anatomical sites, by using disposable equipment, and changing bed linen and collector’s gloves before sampling each subject. During gynecological examination, two cervical smears were collected from each participant for cervical cytological analysis and HPV detection. A cervix brush was used to obtain exfoliated cells from the squamocolumnar junction of the cervix. The collection instrument was then rinsed in transport medium [20, 21]. A collection device (Decipher Bioscience, Shenzhen, China) was used to obtain cervical exfoliated cells for HPV-DNA detection. In accordance with the manufacturer’s instructions, samples were collected by scraping the uterine cervical canal with a cervical brush and sent to a diagnostic laboratory within 24 h. Less than 24 h after birth, exfoliated oral and genital cells were collected from the subset of clean neonates using the same collection device used for HPV DNA detection. Close contact of the child with the mother was avoided to prevent contamination with the mother’s exfoliated cells. Oral samples were obtained by allowing the neonate to suck on the sampler for about 10 s. Genital samples were collected from males by gently swabbing the glans penis and the inner mucosal part of the prepuce. Specimens were obtained from females by gently swabbing the vulvar mucosa. Tips containing cellular material were then placed into transport medium tubes and immediately stored at -20°C.
Laboratory methods
Cytological specimens were sent to a diagnostic pathology laboratory for examination, and prepared using a ThinPrep 2000 Processor (AutoCyte Inc., Burlington, NC, USA), according to the manufacturer’s instructions. Cell suspensions were used to prepare liquid-based cytology slides that were examined using the ThinPrep imager by trained cytologists. The computer-based imaging technology employed for the primary cervical screening for epithelial cell abnormalities used the following classifications [22]: (1) negative for intraepithelial lesion or malignancy (encompassing the previous category of “within normal limits”); (2) atypical squamous or glandular cells of undetermined significance (ASCUS, AGUS); (3) atypical squamous cells, not excluding high-grade squamous intraepithelial lesion (ASC-H); (4) low-grade squamous intraepithelial lesion (LSIL); (5) high-grade squamous intraepithelial lesion (HSIL); and (6) squamous-cell or adenomatous carcinoma (SC, AC). Many studies have confirmed that ThinPrep liquid-based cytology has improved accuracy and reading times, enhancing laboratory productivity and clinical outcomes. This technology also allows HPV testing of the same sample [23, 24].
HPV genotyping was conducted using PCR, DNA hybridization, and an HPV genotyping DNA chip (Decipher Bioscience). An HPV typing kit (Guangdong Hybribio Biotech) was used for quality control. An HPV GenoArray Test Kit (HybrMax), based on “Flow-through Hybridization” and certified for use by the State Food and Drug Administration, was also used for the rapid detection of 21 HPV genotypes. DNA was extracted from samples and centrifuged (13,000 rpm for 10 min). PCR assays were performed in reaction tubes containing 5 μL of DNA and 20 μL of reaction buffer containing specific biotin-tagged oligonucleotide primers. Thermal cycling conditions involved preheating at 50°C for 15 min, then an initial denaturation step at 95°C for 10 min, followed by 40 cycles of 30 s at 94°C, 90 s at 42°C, and 30 s at 72°C, and then a final extension step at 72°C for 5 min. Reverse hybridization was performed using a line-probe assay to enable the PCR products hybridized with specific probes to fix to membranes. Chemical colorization [0.1 M sodium citrate, tetramethylbenzidine (TMB) substrate, 30% H2O2, 30 min] was then conducted to visualize results.
Statistical analyses
Statistical analyses were performed using the SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) to determine factors associated with HPV infection. The influence of delivery type and neonate gender on neonatal HPV infection was analyzed using the χ2 or Fisher’s exact test. A P-value less than 0.005 was considered statistically significant.
Results
The risk factors for HPV infection, and the cervical prevalence of one or more HPV genotypes in the 3139 participants is outlined in Table 1. Overall prevalence of HPV DNA was 13.4% (422/3139). The mean age of uninfected women at delivery was 29.9 ± 3.5 years (mean ± standard deviation; range, 20–44 years), while that of HPV-positive women was 27.9 ± 3.5 years (range, 20–40 years). Prevalence of HPV was significantly higher in women aged 24 years or younger (χ2 = 12.07, P = 0.001). Prevalence of HPV in women experiencing their first pregnancy was 13.8% (234/1692; χ2 = 0.67, P = 0.41). Parity did not appear to be associated with HPV prevalence (OR for ≥3 gestations vs. 1–2 gestations = 1.2, 95% CI: 0.91–1.58). Most women in this study (72.4%) had a level of education that went past secondary school. A significant difference was seen in HPV prevalence among women with different education levels (OR for ≤11 vs. ≥18 years = 4.4, 95% CI: 1.8–11.1; OR for 12–17 years vs. ≥18 years = 32.0, 95% CI: 1.2–7.4; P < 0.01). Abnormal cervical cytological results were found in 42 (1.3%) women, with an HPV prevalence of 76.2% (32/42).
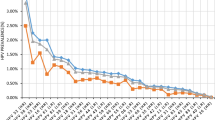
Figure 1 shows the HPV genotypes and frequencies of detection in mothers and neonates. A high proportion of the HPV-positive women (95.5%, 403/422) were infected with high-risk HPV genotypes. The most frequently detected HPV genotypes were HPV-16 (29.6%, 125/422), -18 (14.7%, 62/422), and -58 (14.2%, 60/422). The HPV-16 and -18 genotypes, which can be vaccinated against, accounted for 44.3% (187/422) of HPV infections. Of the 422 HPV-positive samples, 353 (83.6%) contained a single virus genotype, while 69 (16.3%) were positive for two or more HPV genotypes. HPV-16 DNA was present in 46.4% (32/69) of samples that were identified as containing multiple HPV genotypes.
In 23.6% (55/233) of neonates, the oral and/or genital mucosa were positive for HPV DNA. No significant difference was seen between male and female infants (χ2 = 1.64, P = 0.268), or between the type of delivery (χ2 = 0.283, P = 0.71). The HPV-16 genotype accounted for the majority (56.4%, 31/55) of infections in infants. In 233 mother-neonate pairs, the HPV DNA concordance rate between mothers and newborns was 23.6% (55/233). HPV type-specific concordance occurred in 26 cases, with HPV-16 the most frequently detected genotype (18/26), followed by HPV-11 (2/26) and -31 (2/26) (Table 2).
Delivery type did not significantly affect the rate of vertical transmission from HPV-positive mothers, where 58.4% (36/233) of the tested neonates were delivered vaginally and 41.6% (97/233) were delivered by cesarean sections (χ2 = 0.119, P > 0.005). The HPV DNA concordance rate between these 233 mother-neonate pairs was 23.6% (55/233). HPV type-specific concordance occurred in 26 cases (11.2%) and HPV-16 was the most frequently detected genotype (Table 3).
Discussion
In this study we examined the vertical transmission of HPV DNA in a large patient population. Our findings revealed that type-specific HPV discordance was high, suggesting other infection routes besides the maternal-neonate route. Although the prevalence of HPV in mothers with abnormal cervical cytology (76.2%) was much higher than that of mothers with normal cytology, none of the 19 neonates from mothers with abnormal cytology were HPV-positive. There was no significant influence of delivery type on the vertical transmission of HPV, which is consistent with reports of congenital condyloma after cesarean section without premature rupture of membranes [25]. Delivery via cesarean section did not eliminate the risk of vertical transmission, suggesting that such transmission of HPV DNA may occur before birth by the transplacental route. Several studies have found HPV can be found in the amniotic fluid, fetal membranes, and cord blood [26–28]. Disparity of HPV genotypes in maternal and neonatal HPV DNA-positive samples occurred in 52.7% of cases, indicating transmission by fomites (i.e., contaminated instruments), contact with the child after birth, or experimental contamination of the samples might have occurred.
Ping et al. [1] previously reported the detection of HPV-6, -11, -16, and -18 in cervical and vaginal secretions, and in peripheral venous blood samples in women at the latter stages of gestation. Following birth, neonate pharyngeal secretions were also positive for HPV-6, -11, -16, and -18. The prevalence of HPV DNA in the cervical, vaginal exfoliated cells has been shown to be low during the early stages of pregnancy (5/30 cases) and in the second trimester (12/42cases), with prevalence increasing in the final trimester (23/31 cases). In the third trimester, this prevalence was significantly different compared with that during the first two trimesters. Successive examinations on positive women for HPV-6, -11, -16, and -18 in infants at time of birth, 48 ~ 72 h and 6 weeks after birth showed positive HPVDNA in the nasopharyngeal secretion of 13, 6 cases and 1 case with respect to the examining periods. The positive cases were mainly infected by HPV- 16, 18.
Pregnant women in Jiangsu Province usually begin routine obstetric care around the 24th week of gestation; 81.5% of the women in our study were at weeks 22–26 of gestation. Given this small interval of gestational age, it was not possible to examine the influence of this variable on HPV infection in pregnant women. It was previously found that HPV infection in pregnant women was primarily associated with maternal age and education. The influence of education level on HPV infection is most likely explained by the early age of first sexual intercourse and first pregnancy [28].
The microarray used in this study was developed by Decipher Bioscience to detect 23 HPV genotypes. As a diagnostic tool, the application of microarray technology has the advantage of discriminating HPV genotypes and detecting infection of multiple HPV subtypes [29]. In a population-based cross-sectional screening study of 1137 women aged 15–59 years in Shenzhen, Li et al. [30] confirmed the clinical value of this technology for the detection of HPV in cervical cancer screening. Halfo et al. [31] found a 93% concordance (k = 0.82) between the HC-II assay and microarray technology, indicating that microarrays are highly sensitive and specific in the detection of HPV genotypes. The PCR conducted to detect HPV DNA in pregnant women and showed that viral DNA could be detected in exfoliated cervical and vaginal cells. Additionally, this assay also detected DNA for HPV-16, -11, -6, -35, -31, -58. In all cases except one, the HPV DNA detected in the newborn was the same genotype as that detected in the mother [32, 33].
Immunological or hormonal changes may alter the prevalence of HPV and its clearance during pregnancy [34]. Some researchers have reported decreased clearance of high-risk HPV types in the first two trimesters of pregnancy [34, 35], contributing to a high prevalence of HPV during pregnancy. The prevalence of HPV DNA among pregnant women that we observed (13.4%) was similar to that reported by a multicenter epidemiological survey for the general female population of China (13.8%) [36]. Similarly, Zhang et al. [37] found no significant difference between prevalence of HPV DNA and the pregnancy status of 711 women in Beijing.
The prophylactic bivalent HPV 16/18 vaccine manufactured by GlaxoSmithKline is currently in phase-III clinical trials in Jiangsu Province. Few studies have evaluated the safety of this vaccine for pregnant women in China. Given that primary target population of the HPV vaccine is women of a reproductive age, the risks associated with its administration during pregnancy are important factors affecting personal decisions and public health policy. In a study of 3599 pregnancies, Wacholder et al. [15] detected a small increase in the risk of miscarriage for pregnancies conceived within 3 months of vaccination.
The present study found a high prevalence of HPV among pregnant women in Nanjing, the urban center of a highly developed province in China. This prevalence did not differ significantly from that found among the general female population of China. Although some vertical transmission was documented, the high rate of discordance in the HPV genotypes of mothers and neonates indicates the need for efforts to prevent horizontal transmission. Subsequent investigations should examine the role of viral load in vertical transmission.
This large-scale investigation evaluated the prevalence of HPV infection in pregnant women and the rate of vertical transmission. The most frequently detected HPV genotypes in mothers and neonates were HPV-16 and -18, consistent with the target genotypes of HPV vaccines. Currently, no vaccine recommendations for pregnant women have been provided and it is hoped that the current study provides preliminary information that can guide appropriate vaccination strategies for mothers and newborns.
Conclusion
The most frequently detected HPV genotype in pregnant women and newborns was HPV-16.
Ethics approval
This study was approved by the Affiliated Drum Tower Hospital, and Nanjing University Medical School Ethics Committees.
Abbreviations
- HPV:
-
Human papillomavirus
References
Peng P, Weng X, Gu Z: Detection of the Asymptomatic Infection by Human Papillomavirus in Pregnant Women and Neonates. Chinese J Obste Gynecol. 2000, 35: 523-525. http://www.cnki.net/kcms/detail/detail.aspx?dbcode=cjfq&dbname=cjfq2000&filename=zhfc200009004&uid=&p=,
Sigurdsson K, Taddeo FJ, Benediktsdottir KR, Olafsdottir K, Sigvalda-son H, Oddsson K, Rafnar T: HPV genotypes in CIN 2-3 lesions and cervical cancer: a population-based study. Int J Cancer. 2007, 121 (12): 2682-2687. 10.1002/ijc.23034. PubMed PMID:17724723
Cates W: Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999, 26 (4 Suppl): S2-S7. Review. PubMed PMID: 10227693
Jay N, Moscicki AB: Human papillomavirus infections in women with HIV disease: prevalence, risk, and management. AIDS Read. 2000, 10 (11): 659-668. Review. PubMed PMID: 11186191
Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP: Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010, 2010: 326369-Epub 2010 Mar 14. PubMed PMID: 20300545; PubMed Central PMCID: PMC2838362
Armbruster-Moraes E, Ioshimoto LM, Leão E, Zugaib M: Presence of human papillomavirus DNA in amniotic fluids of pregnant women with cervical lesions. Gynecol Oncol. 1994, 54 (2): 152-158. 10.1006/gyno.1994.1185. PubMed PMID: 8063239
Frega A, Cenci M, Stentella P, Cipriano L, De Ioris A, Alderisio M, Vecchione A: Human papillomavirus in virgins and behaviour at risk. Cancer Lett. 2003, 194 (1): 21-24. 10.1016/S0304-3835(03)00048-X. PubMed PMID: 12706855
Watts DH, Koutsky LA, Holmes KK, Goldman D, Kuypers J, Kiviat NB, Galloway DA: Low risk of perinatal transmission of human papillomavirus: results from a prospective cohort study. Am J Obstet Gynecol. 1998, 178 (2): 365-373. 10.1016/S0002-9378(98)80027-6. PubMed PMID: 9500501
de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX: Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007, 7 (7): 453-459. 10.1016/S1473-3099(07)70158-5. Review. PubMed PMID: 17597569
Lin H, Ma YY, Moh JS, Ou YC, Shen SY, ChangChien CC: High prevalence of genital human papillomavirus type 52 and 58 infection in women attending gynecologic practitioners in South Taiwan. Gynecol Oncol. 2006, 101 (1): 40-45. 10.1016/j.ygyno.2005.09.028. Epub 2005 Oct 26. PubMed PMID: 16256180
Ye J, Cheng X, Chen X, Ye F, Lü W, Xie X: Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virol J. 2010, 7: 66-10.1186/1743-422X-7-66. PubMed PMID: 20307327; PubMed Central PMCID: PMC2857835
Medeiros LR, Ethur AB, Hilgert JB, Zanini RR, Berwanger O, Bozzetti MC, Mylius LC: Vertical transmission of the human papillomavirus: a systematic quantitative review. Cad Saude Publica. 2005, 21 (4): 1006-1015. Epub 2005 Jul 11. Review. PubMed PMID: 16021238
LI SQ HONGY, WANG ZQ, HU YL: Screening and management of cervical cytopathology during pregnancy. Jiangsu Medical Journal. 2011, 37 (7): 784-786. http://2010.cqvip.com/QK/93304X/201107/37571620.html,
Fife KH, Katz BP, Brizendine EJ, Brown DR: Cervical human papillomavirus deoxyribonucleic acid persists throughout pregnancy and decreases in the postpartum period. Am J Obstet Gynecol. 1999, 180 (5): 1110-1114. 10.1016/S0002-9378(99)70602-2. PubMed PMID:10329863
Tenti P, Zappatore R, Migliora P, Spinillo A, Belloni C, Carnevali L: Perinatal transmission of human papillomavirus from gravidas with latent infections. Obstet Gynecol. 1999, 93 (4): 475-479. 10.1016/S0029-7844(98)00459-1. PubMed PMID: 10214817
Mant C, Kell B, Rice P, Best JM, Bible JM, Cason J: Buccal exposure to human papillomavirus type 16 is a common yet transitory event of childhood. J Med Virol. 2003, 71 (4): 593-598. 10.1002/jmv.10529. PubMed PMID: 14556274
Smith EM, Ritchie JM, Yankowitz J, Swarnavel S, Wang D, Haugen TH, Turek LP: Human papillomavirus prevalence and types in newborns and parents: concordance and modes of transmission. Sex Transm Dis. 2004, 31 (1): 57-62. 10.1097/01.OLQ.0000105327.40288.DB. PubMed PMID:14695959
Burchell AN, Winer RL, de Sanjosé S, Franco EL: Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006, 24 (Suppl3 S3): 52-61. Epub 2006 Jun 2. Review. PubMed PMID: 16950018
Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, Saah A, Marino D, Ryan D, Radley D, Zhou H, Haupt RM, Garner EI, Quadrivalent Human Papillomavirus Vaccine Phase III Investigators: Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: a combined analysis of five randomized controlled trials. Obstet Gynecol. 2009, 114 (6): 1179-1188. 10.1097/AOG.0b013e3181c2ca21. PubMed PMID: 19935017
Wu X, Huang XH, Zhang WY: Fluid-based thin-layer method for screening of squamous intraepithelial lesions in pregnant women. Zhonghua Fu Chan Ke Za Zhi. 2006, 41 (10): 689-692. Chinese. PubMed PMID: 17199925
Limaye A, Connor AJ, Huang X, Luff R: Comparative analysis of conventional Papanicolaou tests and a fluid-based thin-layer method. Arch Pathol Lab Med. 2003, 127 (2): 200-204.
Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Young N, Forum Group Members: The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002, 287 (16): 2114-2119. 10.1001/jama.287.16.2114. Review. PubMed PMID: 11966386
Chivukula M, Saad RS, Elishaev E, White S, Mauser N, Dabbs DJ: Introduction of the Thin Prep Imaging System (TIS): experience in a high volume academic practice. Cytojournal. 2007, 4: 6-10.1186/1742-6413-4-6. PubMed PMID: 17288596; PubMed Central PMCID: PMC1802998
Davey E, d'Assuncao J, Irwig L, Macaskill P, Chan SF, Richards A, Farnsworth A: Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. BMJ. 2007, 335 (7609): 31-10.1136/bmj.39219.645475.55. Epub 2007 Jun 29. PubMed PMID: 17604301; PubMed Central PMCID:PMC1910624
Meng X-Y, Tao M-F: Feasibility and result of routine screening for cervical disease during gestational period. J Tongji Univ (Medical Science). 2009, 30 (3): 103-105. http://lib.cqvip.com/qk/90060B/200903/30854473.html,
Wang X, Zhu Q, Rao H: Maternal-fetal transmission of human papillomavirus. Chin Med J (Engl). 1998, 111 (8): 726-727.
Tseng CJ, Lin CY, Wang RL, Chen LJ, Chang YL, Hsieh TT, Pao CC: Possible transplacental transmission of human papillomaviruses. Am J Obstet Gynecol. 1992, 166 (1 Pt 1): 35-40.
Louie KS, de Sanjose S, Diaz M, Castellsagué X, Herrero R, Meijer CJ, Shah K, Franceschi S, Muñoz N, Bosch FX, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group: Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer. 2009, 100 (7): 1191-1197. 10.1038/sj.bjc.6604974. Epub 2009 Mar 10. PubMed PMID: 19277042; PubMed Central PMCID: PMC2670004
Kim CJ, Jeong JK, Park M, Park TS, Park TC, Namkoong SE, Park JS: HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol. 2003, 89 (2): 210-217. 10.1016/S0090-8258(02)00069-0.
Li RZ, Shi JF, Zhou QZ, Wu RF, Li N, Wu LN, Zhou YQ, Wang Q, Liu ZH, Liu B, Qiao YL: Evaluation of gene chip technology for high risk type human papillomavirus in cervical cancer screening. Zhonghua Yi Xue Za Zhi. 2006, 86 (5): 307-311. Chinese. PubMed PMID: 16677522
Halfon P, Benmoura D, Khiri H, Penaranda G, Blanc B, Riggio D, Sandri MT: Comparison of the clinical performance of carcinogenic HPV typing of the Linear Array and Papillocheck HPV-screening assay. J Clin Virol. 2010, 47 (1): 38-42. 10.1016/j.jcv.2009.10.013. Epub 2009 Nov 25. PubMed PMID: 19939732
Hong Y, Hu Y, Zhang L, Zhang C, Wang Z, Wu X, Zheng M, Xia Y, Gu Y: Research on the classification and maternal-fetal vertical transmission of human papilloma virus infection in gestational periods. Chin J Pract Gynecol Obstet. 2008, 24 (11): 839-842. http://lib.cqvip.com/qk/90644A/200811/28636784.html,
Yu Y, Hong Y, Jin M, Song S, Hu Y, Wang Z, Shen R, Fang J, Wu X, Li J: A study on mother-to-child transmmission of HPV. Chin J Mat Child Health Res. 2010, 21 (3): 316-319. http://lib.cqvip.com/qk/97998A/201003/34100204.html,
Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, Rozendaal L, Bezemer PD, Voorhorst FJ, Meijer CJ: High-risk human papillomavirus clearance in pregnant women: trends for lower clearance during pregnancy with a catch-up postpartum. Br J Cancer. 2002, 87 (1): 75-80. 10.1038/sj.bjc.6600367. PubMed PMID: 12085260; PubMed Central PMCID:PMC2364279
Qiao YL: Epidemiology of HPV infection and cervical cancer and perspective of HPV vaccination in Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi. 2007, 28 (10): 937-940. Chinese. PubMed PMID: 18399134
Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, Hildesheim A, Rodríguez AC, Solomon D, Herrero R, Schiffman M, CVT group: Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010, 340: c712-10.1136/bmj.c712. PubMed PMID: 20197322; PubMed Central PMCID: PMC2831171
Zhang Y, Li Y, Gao Z: HR-HPV Infection in Pregnant or Postpartum Women. Chin J Nosocomiol. 2009, 11 (10): 1199-1201. http://lib.cqvip.com/qk/98445X/200910/30351921.html,
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/13/109/prepub
Acknowledgements
We thank Yan Yu and Jie Xu for their assistance with the collection of data.
Financial support
This study was supported by Health Bureau of Jiangsu Province (No. H200803), and the Health Bureau of Nanjing (No.YKK05091).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YH conceived and designed the project, and analyzed data. S-QL participated in data collection. Y-LH provided guidance on this project. Z-QW participated in part of the data collection. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hong, Y., Li, SQ., Hu, YL. et al. Survey of human papillomavirus types and their vertical transmission in pregnant women. BMC Infect Dis 13, 109 (2013). https://doi.org/10.1186/1471-2334-13-109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-13-109