Abstract
Background
The purpose of the study is to determine the optical properties and their differences for normal human stomach mucosa/submucosa tissue in the cardiac orifice in vitro at 635, 730, 808, 890 and 980 nm wavelengths of laser.
Methods
The measurements were performed using a CCD detector, and the optical properties were assessed from the measurements using the spatially resolved reflectance, and nonlinear fitting of diffusion equation.
Results
The results of measurement showed that the absorption coefficients, the reduced scattering coefficients, the optical penetration depths, the diffusion coefficients, the diffuse reflectance and the shifts of diffuse reflectance of tissue samples at five different wavelengths vary with a change of wavelength. The maximum absorption coefficient for tissue samples is 0.265 mm-1 at 980 nm, and the minimum absorption coefficient is 0.0332 mm-1 at 730 nm, and the maximum difference in the absorption coefficients is 698% between 730 and 980 nm, and the minimum difference is 1.61% between 635 and 808 nm. The maximum reduced scattering coefficient for tissue samples is 1.19 mm-1 at 635 nm, and the minimum reduced scattering coefficient is 0.521 mm-1 at 980 nm, and the maximum difference in the reduced scattering coefficients is 128% between 635 and 980 nm, and the minimum difference is 1.15% between 890 and 980 nm. The maximum optical penetration depth for tissue samples is 3.57 mm at 808 nm, and the minimum optical penetration depth is 1.43 mm at 980 nm. The maximum diffusion constant for tissue samples is 0.608 mm at 890 nm, and the minimum diffusion constant is 0.278 mm at 635 nm. The maximum diffuse reflectance is 3.57 mm-1 at 808 nm, and the minimum diffuse reflectance is 1.43 mm-1 at 980 nm. The maximum shift Δx of diffuse reflectance is 1.11 mm-1 at 890 nm, and the minimum shift Δx of diffuse reflectance is 0.507 mm-1 at 635 nm.
Conclusion
The absorption coefficients, the reduced scattering coefficients, the optical penetration depths, the diffusion coefficients, the diffuse reflectance and the shifts of diffuse reflectance of tissue samples at 635, 730, 808, 890 and 980 nm wavelengths vary with a change of wavelength. There were significant differences in the optical properties for tissue samples at five different wavelengths (P < 0.01).
Similar content being viewed by others
Background
Knowledge of optical properties for the human stomach mucosa/submucosa tissues in the visible and near infrared (NIR) wavelength range is of great importance in medical applications using light [1, 2], for example, laser coagulation for treatment of early gastric cancer with intramucosal invasion, laser ablation therapy of the submucosal gastric cancer [3], photodynamic ablation therapy of early cancers of the stomach [4], gastrointestinal (GI) diagnosis by the standard white light endoscopy (WLE) and endoscopic diagnosis of premalignant gastrointestinal lesions by fluorescence endoscopic imaging and spectroscopy [5–7], and the recently developed optical coherence tomography (OCT) [8–10] has been reported to image the GI tissues in vitro and in vivo [11–13]. Because of more than 85% of all cancers originate in the epithelia lining the internal surfaces of the human body. The majority of such lesions are readily treatable if diagnosed at an early state [14]. Apart from conventional methods of cancer diagnosis [15–17], there is a need to develop new approaches that are simple, objective, and noninvasive.
The use of optical techniques for gastrointestinal diagnostic purposes relies on the capability to measure the optical properties of gastrointestinal tissue. In recent years, an increasing group of researchers has been interested in nonionizing, near-infrared (NIR) approaches for detecting and imaging diseased tissues. The proposed techniques range from continuous wave [18, 19] to frequency-domain [20, 21] or time-depended measurements of scattered light [22, 23]. These techniques are based on the determination of optical properties of scattering media. The optical properties are represented by the absorption coefficient μa, the scattering coefficient μs and the anisotropy factor g. since the optical detecting and optical imaging are based on selective differences existing in optical properties of healthy and pathological tissues, it is particularly important to diagnostic purpose. For example, Laser-induced autofluorescence (LIAF) spectroscopy has been found to be a promising tool for early cancer diagnosis in gastrointestinal tract, including other organs [24, 25]. Consequently tissue optical properties of healthy and pathological human gastrointestinal tissue are important for medical applications in diagnosis and therapy [26]. We focus in this paper on the optical properties of normal human stomach mucosa/submucosa tissue in the cardiac orifice at the visible and near-infrared wavelength range. The results were analyzed and compared from these experimental data we obtained.
Theory
We utilize a simple two-source diffusion theory model of spatially resolved, steady-state diffuse reflectance [27]. When light enters a semi-infinite tissue, it will generally scatter a number of times before either being absorbed or escaping the tissue surface at a point other than its point of entry. The multiply scattered light that escapes is called diffuse reflectance. Wang and Jacques believe that for both normal and oblique incidence, the more accurate expression for the path length from the tissue surface to the positive point source is what it have been defined as 3D (D is the diffusion coefficient) rather than 1 mfp' (mfp' is the transport mean free path). These two cases were diagrammed in Ref.[28]. The diffuse reflectance profile for oblique incidence is centered about the position of the point sources, the shift Δx by finding the center of diffuse reflectance relative to the light entry point can be measured. As is the case for normal incidence, the diffusion theory model, when shifted by Δx, also agrees with Monte Carlo results outside of 1–2 mfp' from the center of diffuse reflectance, which, it is important to reiterate, is no longer at the point of entry as shown in Ref.[28]. The two-source model, with a depth of 3D instead of 1 mfp', gives the following expression [27, 28]:

which can be scaled arbitrarily to fit a relative reflectance profile that is not in absolute units. Where, μeff is the effective attenuation coefficient, is defined as

ρ 1 and ρ 2 are the distances from the two sources to the point of interest (the point of light collection; see Ref.[28]), and the boundary condition is included in the term A [28]:

where


ntissue is the refractive index of the tissue, nambient is the refractive index of the ambient, and nrel is the relative refractive index of the tissue-air interface. A laser beam is obliquely incident on the top face of the tissue sample, where, θtissue is incident angle of the laser beam. D is the diffusion coefficient, it can be calculated from Δx

where, Δx is the distance between the point of light incidence and the apparent center of diffuse reflectance. According to Lin et al [28] this diffusion constant is equal to

with μs' the reduced scattering coefficient, i.e. μs (1-g), μa the absorption coefficient. The optical properties, μa and μs' were solved from the expressions, and the expressions of μa and μs' are shown as follows


The method to determine tissue optical properties, μa and μs', need sample the relative diffuse reflectance profile at known positions from the light entry point, and need calculate Δx and D, and need perform a nonlinear least-squares fit with the Levenberg-Marquardt method [29–31] on (1) to determine μeff, and then need solve for μa and μs' from the expressions. The method was detailedly shown in Ref.[28].
Methods
Sample preparation
Normal human stomach mucosa/submucosa tissues in the cardiac orifice were investigated in this study. Tissue samples were taken from 12 normal human stomachs in the cardiac orifice were determined from histological examination, immediately after excision the tissues. Each removed stomach sample was immediately rinsed briefly in saline to remove surface excess blood and peeled off surface fats, was placed in a bottle with saline as soon as possible, and was stored in a refrigerator at -70°C. From tissue samples a total of 12 normal stomach mucosa/submucosa tissue samples, with a mean thickness of (10.32 ± 0.26) mm, were used within at most 24 h after remove. The thickness of each sample was measured and recorded with a vernier caliper with 0.02 mm error. All tissue samples were respectively taken out from the refrigerator before measurement, were placed on experimental desk at the room temperature of 20°C for one hour, and then all thawing tissue samples were measured in turn using an oblique incident laser beam and CCD camera, respectively.
Diffuse reflectance measurements of tissue
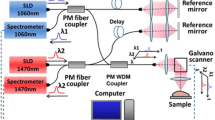
Figure 1 shows a schematic diagram of the experimental setup that is used to measure the relative profile of diffuse reflectance, and table 1 shows information about light source on the experiment. The tissue samples were illuminated with collimated light from 635, 730, 808, 890 and 980 nm wavelength of laser, respectively. The output of all laser light were expanded by the beam expander of 25 times, and then were attenuated (to a power at most 5 mW) by the light attenuators, and were reflected by the mirrors, were passed through a 2 mm pinhole and a 35.2 mm focus of lens, and then the obliquely incident on the top face of the stomach mucosa/submucosa tissue sample at a 45 degree angle between laser axis and the normal to the tissue surface (αi = 45°), respectively. A small piece of transparent ruler (with millimeter gradations) was placed onto the sample surface for scale, and a certain graduation of the ruler was leveled to the center portion of the point of incidence of the laser beam, and the graduation is designated as the origin of the x-coordinate. From the top of the sample a reflectance pattern can be observed. This pattern is imaged on a 795×596 pixel two-dimensional Charge Coupled Device (CCD) detector (Nikon, Cool Pix, 995, Japan). The incident beam can be observed as the most intense area in the image. Because, the laser beam was oblique to the surface the reflectance pattern was asymmetrical near the point of incidence, but the diffuse reflectance far from the source formed concentric circles, approximately, and the distance between the origin of the x-coordinate and the centre of the concentric circles is the distance Δx, and the centre of the concentric circles is also calculated. From the distance Δx the diffusion constant can be calculated using (6), with D the diffusion constant in mm, Δx the distance in mm. This test consisted of repeating ten times reflectance measurements, and the measured results were reproducible for a specific sample at specific wavelength. For each test, the positions of the spot of incident light on the sample surface were altered to decrease the effect of the tissue heterogeneity on the reflectance measurements, and each test at each laser wavelength was performed in the same condition of experimentation, and the exposure time was set at 800 ms. A total of eleven tissue samples were used for the measurements in vitro. The CCD data acquisition were controlled by a computer for the purpose. Data processing and analysis of the data files were performed using custom software written in Matlab (Matlab, Mathworks Incorporated, Massachusetts).
Statistical analysis
Optical parameters of biological tissue samples were expressed as the mean ± SD, were demonstrated by a Student t-test, and were considered significant at p values < 0.01. The SPSS10 was used for the statistical analysis.
Results
The optical properties are expressed as the mean ± SD for all measurements for the samples. Figures 2, 3, 4, 5, 6 and 7 present the wavelength dependence of the absorption coefficients, the reduced scattering coefficients, the optical penetration depths, the diffusion coefficients, the diffuse reflectance and the shifts of diffuse reflectance for normal stomach mucosa/submucosa tissues in the cardiac orifice at five different wavelengths of laser, respectively. The vertical lines correspond to the values of standard deviation (SD), which is determined by a Student t-test, and error bars appear at 635, 730, 808, 890 and 980 nm wavelengths of laser for clarity and represent one standard deviation in the μa, μs', δ, D, R∞ and Δx values.
Discussion
The optical properties of a biological tissue depend on its biochemical composition and its cellular and subcellular structure. In the visible and near-infrared range, the absorption properties are related to the concentration of chromophores, such as oxyhemoglobin and deoxyhemoglobin, fat and water [32]. Such chromophores vary significantly with tissue metabolism [33]. The scattering properties are related to the size distribution of cells and organelles, which are parameters used to differentiate normal from abnormal tissues in standard histopathology [34]. Therefore optical measurements have a strong potential for the development of noninvasive in vivo medical diagnostic tools, often called "optical biopsy". Such techniques should significantly improve the efficiency of biopsies or help in determining the tumor margins in a surgical field. According to our experimental data, the absorption coefficients, the reduced scattering coefficients, the optical penetration depths, the diffusion coefficients, the diffuse reflectance and the shifts of diffuse reflectance for normal stomach mucosa/submucosa tissues in the cardiac orifice at 635, 730, 808, 890 and 980 nm were determined in vitro. In our study, it is interesting to note the optical properties measured and their differences for the tissue samples at five different laser wavelengths. We believe the optical properties should help to pathological diagnosis and medical treatment for malignant or premalignant gastrointestinal mucosa with ease by using optical methods.
Figure 2 and Figure 3 show the absorption coefficients and the reduced scattering coefficients of tissue samples at five different laser wavelengths, respectively. From Figure 2 and Figure 3, it can be seen that the absorption coefficients for tissue samples increase with the increase of laser wavelengths, except for the absorption coefficient at 730 nm, and the reduced scattering coefficients for tissue samples decrease with the increase of laser wavelengths. There were significant differences in the absorption coefficients at five different laser wavelengths (P < 0.01). The maximum and the minimum absorption coefficients are 0.265 mm-1 at 980 nm and 0.0332 mm-1 at 730 nm, respectively. The maximum and the minimum differences of the absorption coefficients are 698% between 730 and 980 nm and 1.61% between 635 and 808 nm, respectively. There also were significant differences in the reduced scattering coefficients at five different laser wavelengths (P < 0.01). The maximum and the minimum reduced scattering coefficients are 1.19 mm-1 at 635 nm and 0.521 mm-1 at 980 nm, respectively. The maximum and the minimum differences of the reduced scattering coefficients are 128% between 635 and 980 nm and 1.15% between 890 and 980 nm, respectively.
Figure 4 shows that the optical penetration depths for tissue samples vary with the increase of laser wavelengths. There were significant differences in the optical penetration depths at five different laser wavelengths (P < 0.01). The maximum and the minimum optical penetration depths are 3.57 mm at 808 nm and 1.43 mm at 980 nm, respectively. The maximum and the minimum differences of the optical penetration depths are 150% between 808 and 980 nm and 5.36% between 730 and 890 nm, respectively. From Figure 5, it can be seen that the diffusion coefficients for tissue samples vary with the increase of laser wavelengths. There also were significant differences in the diffusion coefficients at five different laser wavelengths (P < 0.01). The maximum and the minimum diffusion coefficients are 0.608 mm-1 at 890 nm and 0.278 mm-1 at 635 nm, respectively. The maximum and the minimum differences of the diffusion coefficients are 119% between 635 and 890 nm and 12.0% between 890 and 980 nm, respectively. Figure 6 shows that the diffuse reflectance for tissue samples decrease with the increase of laser wavelengths. There were significant differences in the diffuse reflectance at five different laser wavelengths (P < 0.01). The maximum and the minimum diffuse reflectance are 0.456 at 635 nm and 0.0732 at 980 nm, respectively. The maximum and the minimum differences of the diffuse reflectance are 523% between 635 and 980 nm and 7.29% between 635 and 730 nm, respectively. From Figure 7, it can be seen that the shift Δx of diffuse reflectance for tissue samples vary with the increase of laser wavelengths. There also were significant differences in the shift Δx of diffuse reflectance at five different laser wavelengths (P < 0.01). The maximum and the minimum shift Δx of diffuse reflectance are 1.11 mm at 890 nm and 0.507 mm at 635 nm, respectively. The maximum and the minimum differences of the shift Δx of diffuse reflectance are 119% between 635 and 890 nm and 11.7% between 890 and 980 nm, respectively.
There are significant differences in the optical properties of the tissue samples between different wavelengths of laser (P < 0.01). Bashkatov, et al.[35] and Holmer et al.[36] have reported the optical properties of gastric tissue by different optical measurement methods, our data that the wavelength dependence of the absorption coefficient, the reduced scattering coefficient and the optical penetration depth of human stomach wall mucosa are very similar to compare the data of Bashkatov, et al. and Holmer et al. with our data in the spectral range from 600 to 1000 nm.
Conclusion
In conclusion, the results reported here indicate that differences in the optical properties, namely, the absorption coefficients, the reduced scattering coefficients, the optical penetration depths, the diffusion coefficients, the diffuse reflectance and the shifts of diffuse reflectance for normal stomach mucosa/submucosa tissues in the cardiac orifice at 635, 730, 808, 890 and 980 nm are significant in vitro (P < 0.01), and the potential and promise of using an oblique incident laser beam to measure the optical properties of tissue for clinical studies. Tissues of various pathologies have differing optical tissue properties, and tissues of different places for normal human stomachs have differing optical tissue properties [2]. The preliminary results presented can be used for the development of optical technologies and can be useful in earlier diagnosis, photodynamic and photothermal therapy in the gastrointestinal tract.
Abbreviations
- NIR:
-
near infrared
- GI:
-
gastrointestinal
- WLE:
-
white light endoscopy
- OCT:
-
optical coherence tomography
- LIAF:
-
laser-induced autofluorescence
- mfp':
-
the transport mean free path
- D:
-
the diffusion coefficient
- SD:
-
standard deviation
References
Lovat LB, Bown SG: Lasers in gastroenterology. World J Gastroenterol. 2001, 7 (3): 317-323.
Thueler P, Charvet I, Bevilacqua F, Ghislain MS, Ory G, Marquet P, Meda P, Vermeulen B, Depeursinge C: In vivo endoscopic tissue diagnostics based on spectroscopic absorption, scattering, and phase function properties. J Biomed Opt. 2003, 8 (3): 495-503. 10.1117/1.1578494.
Hayashi T, Arai T, Tokonabe S, Itoh H, Kikuchi M, S. Hino S, Masuda K, Suzuki H, Tajiri H, Hino K, Nogami Y: Diode-laser ablation therapy for the submucosal gastric cancer using indocyanine green solution injection to the submucosa. Proc SPIE. 1997, 2975: 408-414. full_text.
Ell C, Gossner L, May A, Schneider HT, Hahn EG, Stolte M: Photodynamic ablation of early cancers of the stomach by means of mTHPC and laser irradiation: preliminary clinical experience. Gut. 1998, 43 (3): 345-349.
DaCosta RS, Wilson BC, Marcon NE: New optical technologies for earlier endoscopic diagnosis of premalignant gastrointestinal lesions. J Gastroenterol Hepatol. 2002, 17 (Suppl): S85-S104. 10.1046/j.1440-1746.17.s1.8.x.
Silveira L, Filho JAB, Silveira FL, Zângaro RA, Pacheco MTT: Laser-Induced Fluorescence at 488 nm Excitation for Detecting Benign and Malignant Lesions in Stomach Mucosa. J Fluoresc. 2008, 18: 35-40. 10.1007/s10895-007-0232-y.
Friedland S, Soetikno R, Benaron D: Reflectance spectrophotometry for the assessment of mucosal perfusion in the gastrointestinal tract. Gastrointest Endoscopy Clin N Am. 2004, 14 (3): 539-553. 10.1016/j.giec.2004.03.011.
Levitz D, Thrane L, Frosz MH, Andersen PE, Andersen CB, Andersson-Engels S, Valanciunaite J, Swartling J, Hansen PR: Determination of optical scattering properties of highly-scattering media in optical coherence tomography images. Opt Express. 2004, 12 (2): 249-259. 10.1364/OPEX.12.000249.
Kuranov RV, Sapozhnikova VV, Turchin IV, Zagainova EV, Gelikonov VM, Kamensky VA: Complementary use of cross-polarization and standard OCT for differential diagnosis of pathological tissues. Opt Express. 2002, 10 (15): 707-713.
Wang RK: Signal degradation by multiple scattering in optical coherence tomography of dense tissue: a Monte Carlo study towards optical clearing of biotissues. Phys Med Biol. 2002, 47 (13): 2281-2299. 10.1088/0031-9155/47/13/307.
Wang RK, Xu XQ, He YH, Elder JB: Investigation of optical clearing of gastric tissue immersed with hyperosmotic agents. IEEE J Sel Top Quantum Electron. 2003, 9 (2): 234-242. 10.1109/JSTQE.2003.813300.
Xu XQ, Wang RK, Elder JB: Optical clearing effect on gastric tissues immersed with biocompatible chemical agents investigated by near infrared reflectance spectroscopy. J Phys D: Appl Phys. 2003, 36 (14): 1707-1713. 10.1088/0022-3727/36/14/309.
Xu XQ, Wang RK: Synergistic effect of hyperosmotic agents of dimethyl sulfoxide and glycerol on optical clearing of gastric tissue studied with near infrared spectroscopy. Phys Med Biol. 2004, 49 (3): 457-468. 10.1088/0031-9155/49/3/008.
Kim YL, Liu Y, Wali RK, Roy HK, Goldberg MJ, Kromin AK, Chen K, Vadim B: Simultaneous measurement of angular and spectral properties of light scattering for characterization of tissue microarchitecture and its alteration in early precancer. IEEE J Sel Top Quantum Electron. 2003, 9 (2): 243-256. 10.1109/JSTQE.2003.814183.
Mourant JR, Bigio IJ, Boyer J, Johnson TM, Lacey J, Bohorfoush AG, Mellow M: Elastic scattering spectroscopy as a diagnostic tool for differentiating pathologies in the gastrointestinal tract: preliminary testing. J Biomed Opt. 1996, 1 (2): 192-199. 10.1117/12.231372.
Keijzer M, Richards-Kortum RR, Jacques SL, Feld MS: Fluorescence spectroscopy of turbid media: Autofluorescence of the human aorta. Appl Opt. 1989, 28 (20): 4286-4292. 10.1364/AO.28.004286.
Mourant JR, Freyer JP, Hielscher AH, Eick AA, Shen D, Johnson TM: Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics. Appl Opt. 1998, 37 (16): 3586-3593. 10.1364/AO.37.003586.
Mourant JR, Hielscher AH, Eick AA, Johnson TM, Freyer JP: Evidence of intrinsic differences in the light scattering properties of tumorigenic and nontumorigenic cells. Cancer Cytopathol. 1998, 84 (6): 366-374.
Swartling J, Dam JS, Andersson-Engels S: Comparison of spatially and temporally resolved diffuse-reflectance measurement systems for determination of biomedical optical properties. Appl Opt. 2003, 42 (22): 4612-4620. 10.1364/AO.42.004612.
Shah N, Cerussi A, Eker C, Espinoza J, Butler J, Fishkin J, Hornung R, Tromberg B: Noninvasive functional optical spectroscopy of human breast tissue. Proc Natl Acad Sci USA. 2001, 98 (8): 4420-4425. 10.1073/pnas.071511098.
Shah N, Cerussi AE, Jakubowski D, Hsiang D, Butler J, Tromberg BJ: Spatial variations in optical and physiological properties of healthy breast tissue. J Biomed Opt. 2004, 9 (3): 534-540. 10.1117/1.1695560.
Kienle A, Glanzmann T: In vivo determination of the optical properties of muscle with time-resolved reflectance using a layered model. Phys Med Biol. 1999, 44 (11): 2689-2702. 10.1088/0031-9155/44/11/301.
Yu P, Peng L, Mustata M, Turek JJ, Melloch MR, Nolte DD: Time-dependent speckle in holographic optical coherence imaging and the health of tumor tissue. Opt Lett. 2004, 29 (1): 68-70. 10.1364/OL.29.000068.
Huang ZW, Zheng W, Xie SS, Chen R, Zeng HS, McLean DI, Lui H: Laser-induced autofluorescence microscopy of normal and tumor human colonic tissue. Int J Oncol. 2004, 24 (1): 59-63.
Marchesini R, Brambilla M, Pignoli E, Bottiroli G, Croce AC, Dal Fante M, Spinelli P, di Palma S: Light-induced fluorescence spectroscopy of adenomas, adenocarcinomas and nonneoplastic mucosa in human colon. I. In vitro measurements. J photochem photobiol B Biol. 1992, 14 (3): 219-230. 10.1016/1011-1344(92)85100-9.
Pitris C, Jesser C, Boppart SA, Stamper D, Brezinski ME, Fujimoto JG: Feasibility of optical coherence tomography for high-resolution imaging of human gastrointestinal tract malignancies. J Gastroenterol. 2000, 35 (2): 87-92. 10.1007/s005350050019.
Farrell TJ, Patterson MS, Wilson BC: A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the non-invasive determination of tissue optical properties in vivo. Med Phys. 1992, 19 (4): 879-888. 10.1118/1.596777.
Lin SP, Wang LH, Jacques SL, Tittel FK: Measurement of tissue optical properties by the use of oblique-incidence optical fiber reflectometry. Appl Opt. 1997, 36 (1): 136-143. 10.1364/AO.36.000136.
Marquardt DW: An algorithm for least-squares estimation of nonlinear parameters. J Soc Indust Appl Math. 1963, 11 (2): 431-441. 10.1137/0111030.
Levenberg K: A Method for the Solution of Certain Non-Linear Problems in Least Squares. Journal of Applied Mathmatics. 1944, 11 (2): 164-168.
Gill PE, Murray W: Algorithms for the solution of the nonlinear least-squares problem. SIAM Journal on Numerical Analysis. 1978, 15 (5): 977-992. 10.1137/0715063.
Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ: Broadband absorption spectroscopy in turbid media by combining frequency-domain and steady-state methods. Appl Opt. 2000, 39 (34): 6498-6507. 10.1364/AO.39.006498.
Cerussi AE, Berger AJ, Bevilacqua F, Shah N, Jakubowski D, Butler J, Holcombe RF, Tromberg BJ: Sources of contrast for quantitative non-invasive optical spectroscopy of breast tissue physiology. Acad Radiol. 2001, 8 (3): 211-218. 10.1016/S1076-6332(03)80529-9.
Perelman LT, Backman V, Wallace M, Zonios G, Manoharan R, Nuserat A, Shields S, Seiler M, Lima C, Hamano T, Itzkan I, Van Dam J, Crawford JM, Feld MS: Observation of periodic fine structure in reflectance from biological tissue: a new technique for measuring nuclear size distribution. Phys Rev Lett. 1998, 80 (3): 627-630. 10.1103/PhysRevLett.80.627.
Bashkatov AN, Genina EA, Kochubey VI, Gavrilova AA, Kapralov SV, Grishaev VA, Tuchin VV: Optical properties of human stomach mucosa in the spectral range from 400 to 2000 nm: prognosis for gastroenterology. Medical Laser Application. 2007, 22 (2): 95-104. 10.1016/j.mla.2007.07.003.
Holmer C, Lehmann KS, Wanken J, Reissfelder C, Roggan A, Mueller G, Buhr HJ, Ritz JP: Optical properties of adenocarcinoma and squamous cell carcinoma of the gastroesophageal junction. J Biomed Opt. 2007, 12 (1): 014025-1-014025-8. 10.1117/1.2564793.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/9/64/prepub
Acknowledgements
The authors would like to acknowledge the National Natural Science Foundation of China (item number 30470494; 30627003), and the Natural Science Foundation of Guangdong Province (item number 7117865) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HW has been involved in the design and conception of the study, supervision of the work, acquisition and analysis of data, and writing the manuscript. DX contributed in the design of the study, supervised the study. BH participated in the histological studies, carried out gastric biopsies, and performed the statistical analysis. HG coordinated the study. GW participated in the histological studies, and carried out gastric biopsies, and performed the statistical analysis. XC participated in the histological studies, and carried out gastric biopsies, and performed the statistical analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wei, H.J., Xing, D., He, B.H. et al. Using an oblique incident laser beam to measure the optical properties of stomach mucosa/submucosa tissue. BMC Gastroenterol 9, 64 (2009). https://doi.org/10.1186/1471-230X-9-64
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-9-64