Abstract
Background
The purpose of the Occluded Artery Trial (OAT) Biomarker substudy was to evaluate the impact of infarct related artery (IRA) revascularization on serial levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and dynamics of other biomarkers related to left ventricular remodeling, fibrosis and angiogenesis.
Methods
Patients were eligible for OAT-Biomarker based on the main OAT criteria. Of 70 patients (age 60.8 ± 8.8, 25% women) enrolled in the substudy, 37 were randomized to percutaneous coronary intervention (PCI) and 33 to optimal medical therapy alone. Baseline serum samples were obtained prior to OAT randomization with follow up samples taken at one year. The primary outcome was percent change of NT-proBNP from baseline to 1 year. The secondary outcomes were respective changes of matrix metalloproteinases (MMP) 2 and 9, tissue inhibitor of matrix metalloproteinase 2 (TIMP-2), Vascular Endothelial Growth Factor (VEGF), and Galectin-3.
Results
Paired (baseline and one-year) serum samples were obtained in 62 subjects. Baseline median NT-proBNP level was 944.8 (455.3, 1533) ng/L and decreased by 69% during follow-up (p < 0.0001). Baseline MMP-2 and TIMP-2 levels increased significantly from baseline to follow-up (p = 0.034, and p = 0.027 respectively), while MMP-9 level decreased from baseline (p = 0.038). Levels of VEGF and Galectin-3 remained stable at one year (p = NS for both). No impact of IRA revascularization on any biomarker dynamics were noted.
Conclusions
There were significant changes in measured biomarkers related to LV remodeling, stress, and fibrosis following MI between 0 and 12 month. Establishing infarct vessel patency utilizing stenting 24 hours-28 days post MI did not however influence the biomarkers’ release.
Similar content being viewed by others
Background
Myocardial infarction (MI) initiates healing and repair responses, which include scar formation, angiogenesis and remodeling. Preliminary data suggested that late (beyond 24 hours) opening of occluded culprit infarct artery might have beneficial impact upon these processes, with subsequent clinical advantages [1]. The Occluded Artery Trial (OAT) was a randomized trial testing the clinical effect of mechanical reperfusion versus optimal medical therapy alone (MED) for totally occluded arteries in stable patients 3–28 calendar days following MI. The main trial was powered to evaluate clinical outcomes, and failed to show any additional benefit of the interventional treatment over optimal medical therapy [2]. The lack of observed clinical benefit with percutaneous coronary intervention (PCI) was also independent of the presence and degree of viability in the infarct zone [3]. Changing plasma levels of relevant biomarkers may reflect the dynamics of these processes more sensitively, and their assessment may provide additional pathophysiological insight into the main study clinical findings.
The primary purpose of the OAT Biomarker substudy was to evaluate the impact of mechanical revascularization on paired levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) obtained at baseline and at 1 year of follow up; and secondly to assess the impact of the randomized treatment on dynamics of biomarkers related to post-infarction left ventricular remodeling, fibrosis and angiogenesis, including matrix metalloproteinases (MMP) 2 and 9 and tissue inhibitor of matrix metalloproteinase 2 (TIMP-2), Vascular Endothelial Growth Factor (VEGF), and galectin-3.
Methods
Study setting & population
The patients were eligible for OAT-Biomarker ancillary study when they consented for the parent trial OAT (ClinicalTrials.gov Identifier: NCT00004562). Due to lack of funding the study was performed in only 10 sites in Poland, two in Canada and a solitary site in the United States. The substudy was approved by Institutional Review Board at each participating center (Terenowa Komisja Bioetyczna Instytutu Kardiologii; University Health Network Research Ethics Board; Research Ethics Board, St. Michael’s Hospital; Ethics Committee, Truman Medical Center) and patients provided separate written informed consent for the OAT-Biomarker ancillary study. During follow-up repeated blood sampling 1 year after randomization was performed. For ninety percent (62/70) of the patients paired (baseline plus on-year) blood samples were available.
Laboratory methods and study outcomes
Baseline samples were obtained prior to OAT randomization and repeated after one year. Blood samples were collected in tubes without anticoagulant. The samples were then centrifuged, and serum was stored frozen in aliquots at -20°C to -80°C at the enrolling site until shipped to the biomarker core laboratory at the Institute of Cardiology, where they were maintained at -80°C. Serum NT-proBNP (sandwich immunoassay) for this batched analysis were determined using an Elecsys 2010 (Roche Diagnostics GmbH, Mannheim, Germany). The analytic range of NT-proBNP assay extends from 5 to 35000 ng/L. The total coefficient of variation (CV) was 2.1% at a level of 181 ng/L and 1.8% at a level of 572 ng/L. Concentrations of matrix metalloproteinases, tissue inhibitor of metalloproteinase-2 and VEGF were assayed using quantitative sandwich enzyme immunoassay technique. The following Quantikine (R&D Systems, Inc., Minneapolis, MN, USA) tests were used: human/mouse MMP-2, total (sensitivity =0.16 ng/mL, intra- and inter-assay precision; CV = 5.7 and 8.2%), human MMP-9, total, (sensitivity = 0.156 ng/mL, intra- and inter-assay precision; CV = 2.9 and 7.9%), human TIMP-2 (sensitivity = 0.011 ng/mL, intra- and inter-assay precision; CV = 4.4 and 7.3%), human VEGF (sensitivity =9.0 pg/mL, intra- and inter-assay precision; CV = 4.5 and 7.0%). Concentration of galectin was estimated using human galectin-3 ELISA kit (Bender MedSystems GmbH, Vienna, Austria) (sensitivity = 0.12 ng/mL, intra- and inter-assay precision; CV = 6.4 and 11.4%).
The primary endpoint for this biomarker analysis was NT-proBNP percent change from baseline to 1 year. The dynamics of the other biomarkers comprised the secondary outcomes of the study. The primary clinical endpoint of OAT was a composite of death from any cause, reinfarction, or NYHA class IV heart failure with hospitalization or admission in a short-stay unit with follow up extending out to 7 years. The study clinical endpoints were adjudicated by an independent committee. The definition of reinfarction has been provided previously [2].
Statistical analysis
Data management and statistical analyses were performed by the OAT data coordinating center. Baseline biomarkers were assessed as medians (interquartile range), clinical characteristics were assessed as frequencies/proportions or mean ± standard deviation (SD) and compared to the remaining main OAT patients, as well as by treatment groups (PCI vs. MED). The nonparametric median Wilcoxon test, t-test or chi square tests were used for comparisons as appropriate. The biomarker’s levels were presented as medians (inter-quartile range), and compared by means of Wilcoxon Signed Rank test. Two-sided two-group t-tests at alpha = 0.05 were done for comparisons of biomarkers changes from baseline to 1-year.
To examine the impact of the study treatment on biomarker dynamics, change in biomarkers’ levels between baseline and 1 year were compared by treatment assignment (PCI versus MED). The independent correlates of NT-proBNP and other biomarkers dynamics (change from baseline to one year levels) were assessed by means of regression analyses. The univariate models were run first to identify any predictor significant at 0.10 level. Then a backward selection was run on the multivariate model to determine the final predictors that were still significant at 0.05 level. The treatment group was forced into the multivariable models. For the biomarkers dynamics (baseline versus follow-up), biomarkers dynamics between PCI versus MED subgroups, and for regression models with NT-proBNP dynamics as the dependent variable, significance level was set at p < 0.05. For other analyses significance level was set at p < 0.01. All analyses are performed on an intention-to-treat basis.
With a targeted sample size of 200 patients and a predicted standard deviation of 50% for change in biomarker levels from baseline to 1 year, there would be 80% power to detect a 22% difference in NT-proBNP change between the treatment groups in the presence of a 15% drop-out rate. With the observed SD of 76% and sample size of 62 (paired samples), there was 80% power to detect a difference of 56% for the primary outcome of change in NT-proBNP levels from baseline to 1 year.
Results
Baseline characteristics
Patient characteristics at study baseline and comparison to the main OAT cohort are illustrated in Table 1. The mean age of enrolled patients was 60.8 ± 8.8 years, all patients were white (p < 0.001 when compared to the main OAT), and 17 (25%) were women. Patients enrolled in the biomarker substudy had a longer time from MI to randomization, less thrombolytic therapy for the index event, lower prevalence of multivessel disease, and higher use of lipid lowering medications and spironolactone (Table 1). Overall, thirty seven biomarker study subjects were randomized to PCI while 33 were in the control arm. Patients randomized to medical therapy alone had less diabetes and received less thienopyridines, when compared to the PCI group (Table 2).
Biomarkers
Baseline levels of NT-proBNP were 944.8 (455.3, 1533) ng/L and decreased by 69% to 266.8 (138.2, 584.9) ng/L during follow-up (p < 0.0001). During this period MMP-2 [185.7(172.3, 219.4) ng/mL versus 216.9 (176, 249.9) ng/mL, p = 0.034] and TIMP-2 levels [64.5 (58.6, 71) ng/mL versus 69.1 (62.6, 74.4) ng/mL, p = 0.027] increased significantly from baseline to follow-up. In contrast, MMP-9 levels decreased from 101.6 (53.5, 170.2) ng/mL to 80.2 (43.7, 150.2) ng/mL (p = 0.038) while levels of VEGF [39.9 (21.7, 83.7) pg/mL vs. 48.3 (19.6, 82.5) p = 0.291], and Galectin [12.8 (9.4, 15.2) ng/mL vs. 12.8 (10.1, 15.4) ng/mL, p = 0.561] remained stable (Figure 1).
Impact of PCI on biomarker levels
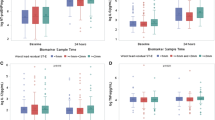
The percent change from baseline to one year levels of NT-proBNP was significant within both PCI and MED subgroups. There was a trend toward increased MMP-2 in medically treated patients only, and MMP-9 tended to decrease in PCI but not in medically treated patients (Figure 2). Follow-up levels of the examined biomarkers and their changes over time did not differ significantly for the PCI versus MED subgroups (Figure 2).
Baseline to one-year changes of the examined biomarkers’ levels by the study treatment (recanalization of infarct related artery (PCI) versus medical therapy alone (MED)). (A) - NT-proBNP- N-terminal prohormone of brain natriuretic peptide; (B) - VEGF-Vascular Endothelial Growth Factor; (C) - MMP-2-matrix metalloproteinase-2; (D) - MMP-9-matrix metalloproteinase-9; (E) - TIMP-2 - tissue inhibitor of matrix metalloproteinase-2; (F) - Galectin.
Predictors of the biomarkers’ dynamics
The univariate and multivariable predictors of biomarker changes over time are provided in Table 3. Percent change of NT pro-BNP was predicted independently by its baseline level and family history of coronary disease. For the other biomarkers, their dynamics were independently predicted by their respective baseline values. Additionally, changes in VEGF were correlated to previous thrombolytic therapy; while changes in TIMP-2 were related to history of renal insufficiency, baseline heart rate and BMI. Galectin changes were related to BMI (Table 3).
Discussion
This is a small, serial measurement of biomarkers substudy performed under the auspices of a large phase 3 rigorously performed randomized clinical trial. Despite its small size, the unique patient characteristics and the protocol assigned PCI in the background of a late totally occluded infarct related artery makes these observations notable. The differences in baseline characteristics from the parent trial likely reflect geographic characteristics arising from randomization in Poland which accounted for all of the paired samples considered for analysis. Prior analyses of the main trial demonstrated similar treatment effect across regions. Our study demonstrated that the plasma levels of biomarkers related to LV function and remodeling, including NT-proBNP, MMP-2, TIMP-2 and MMP-9, significantly evolved from baseline to one year following index MI. However, the change in biomarkers levels were not significantly modified by mechanical opening of the occluded infarct related artery (IRA) performed 3 to 28 days post MI.
NT-proBNP is an established biomarker used for diagnosis, prognosis and in selected cases management of patients with heart failure or acute coronary syndromes. It is synthesized in the ventricular myocardium in response to increased wall tension, myocardial stretch or ischemia [4–6]. The kinetics of NT-proBNP in the early post infarction period have been previously reported. An early rise is seen beginning immediately after the index infarct that has a second peak at about 24 hours. Subsequently NT-proBNP levels plateau at approximately 35 days. Plasma levels of NTpro-BNP chave been shown to correlate with cardiac function following myocardial infarction [7], as well as with mortality in heart failure or acute coronary syndromes. Serial changes of NT-proBNP parallel improving or worsening LV function [8, 9]. In a substudy of FRISC-II trial, higher baseline levels of NT-proBNP were shown to identify the subgroup of NSTE-ACS patients benefitting from interventional treatment [10]. The decline of NT-proBNP observed in OAT-Biomarker likely reflects gradual improvement of cardiac function (ejection fraction) directly observed in OAT-NUC and TOSCA 2 ancillary studies [3, 11]. We observed a lack of impact of the IRA recanalization on biomarker changes over one year. These findings are consistent with the lack of clinical benefit observed with PCI in the parent trial. Importantly, majority (89%) of the study patients received ACE inhibitors or angiotensin receptor-1 blockers, therefore the hypothetical impact of the study PCI was tested on top of optimal medical therapy, which itself had been shown to lower natriuretic peptide levels in patients with LV dysfunction [12].
Matrix metalloproteinases (MMP) and their tissue inhibitors (TIMPs) are enzymes involved in the extracellular matrix turnover and cardiac remodeling following infarction. Their release and roles following MI are time dependent [13, 14]. Prior studies reported a cause-effect relation between MMP-2,-9 and adverse LV myocardial remodeling after MI [15, 16]. MMP-2 and -9 levels were shown to correlate with echo measures of LV remodeling and non scarred myocardial mass on CMR [17–19]. Existing evidence supports clinical testing of MMP inhibitors for prevention of postinfarction remodeling in humans [13, 20].
In our cohort the values of MMP-2 significantly increased, while MMP-9 decreased from baseline. Similar shifts in these biomarker levels during long-term follow-up were previously observed in patients after MI [17, 18, 21, 22]. In our study, PCI tended to decrease MMP-9 levels more significantly than medical therapy alone (respectively 38% vs. 5%). Regarding MMP-2, PCI had no visible effect on its dynamics.
TIMP-2 may either inhibit or stimulate activation of MMP-2 [23, 24]. Its deficiency has been shown to accelerate post infarction LV remodeling in an animal model [25]. Plasma levels of TIMP-2 have been shown to increase in late stages of MI, as we observed in our cohort [17]. In the current study, TIMP-2 rise was positively correlated with history of renal insufficiency and heart rate and negatively with body mass index. However, PCI of IRA did not significantly impact alterations in these marker dynamics.
VEGF is implicated in neovascularization of ischemic tissue and wound healing, and its levels were previously shown to be correlated with peak creatine kinase levels in patients with MI. VEGF elevation has also been shown to be associated with clinical myocardial ischemia and adverse outcomes following ACS [26, 27]. The only independent correlate of the VEGF dynamics in our study was thrombolytic therapy for the index event. We did not find any association between the study treatment and baseline to one-year change of VEGF levels.
Experimental observations suggest that galectin-3 may be regarded not only as a biomarker but also as an active mediator of fibrosis causing progression of heart failure [28, 29]. In the clinical setting galectin-3 levels have been shown to correlate with clinical outcomes in patients with heart failure, providing incremental prognostic value to NT-proBNP [30]. In our study no significant difference was found between median baseline and follow up values of galectin, nor between PCI and medically treated patients. However, multivariable analysis identified thrombolytic therapy and BMI as independent correlates of the biomarker dynamics over one-year.
Limitations
The study cohort represents a small series of patients enrolled in the main OAT study. The power to detect small differences in the biomarker dynamics between the PCI and MED groups was limited due to the insufficient recruitment relative to initial projections. It is also conceivable that the differences between the study groups would differ had the patients been followed for a longer period of time. Only paired samples at baseline and 1 year of follow up were available. As a result the time course of changes during this window could not be elicited.
Conclusions
The Biomarker ancillary study to OAT suggests that there are significant dynamic changes (from baseline to one year) in biomarkers related to LV remodeling, stress, and fibrosis following MI, however, their dynamics are not significantly modified by opening of occluded IRA 3 days-28 days post MI. Consistent with the findings of the parent trial and the nuclear substudy, our findings appear to also suggest that late IRA recanalization does not significantly impact mechanisms underlying LV healing/remodeling processes following MI in patients on optimal medical therapy.
References
Sadanandan S, Buller C, Menon V, Dzavik V, Terrin M, Thompson B, Lamas G, Hochman JS: The late open artery hypothesis–a decade later. Am Heart J. 2001, 142: 411-421. 10.1067/mhj.2001.117774.
Hochman JS, Lamas GA, Buller CE, et al: Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006, 355: 2395-2407. 10.1056/NEJMoa066139.
Udelson JE, Pearte CA, Kimmelstiel CD, et al: The Occluded Artery Trial (OAT) Viability Ancillary Study (OAT-NUC): influence of infarct zone viability on left ventricular remodeling after percutaneous coronary intervention versus optimal medical therapy alone. Am Heart J. 2011, 161: 611-621. 10.1016/j.ahj.2010.11.020.
Levin ER, Gardner DG, Samson WK: Natriuretic peptides. N Engl J Med. 1998, 339: 321-328. 10.1056/NEJM199807303390507.
Goetze JP, Christoffersen C, Perko M, et al: Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003, 17: 1105-1107.
Goetze JP, Gore A, Møller CH, Steinbrüchel DA, Rehfeld JF, Nielsen LB: Acute myocardial hypoxia increases BNP gene expression. FASEB J. 2004, 18: 1928-1930.
Richards AM, Nicholls MG, Yandle TG, et al: Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998, 97: 1921-1929. 10.1161/01.CIR.97.19.1921.
Gackowski A, Isnard R, Golmard JL, et al: Comparison of echocardiography and plasma B-type natriuretic peptide for monitoring the response to treatment in acute heart failure. Eur Heart J. 2004, 25: 1788-1796. 10.1016/j.ehj.2004.07.038.
Michtalik HJ, Yeh HC, Campbell CY, et al: Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. Am J Cardiol. 2011, 107: 1191-1195. 10.1016/j.amjcard.2010.12.018.
Jernberg T, Lindahl B, Siegbahn A, et al: N-terminal pro-brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. J Am Coll Cardiol. 2003, 42: 1909-1916. 10.1016/j.jacc.2003.07.015.
Dzavík V, Buller CE, Lamas GA, et al: Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation. 2006, 114: 2449-2457. 10.1161/CIRCULATIONAHA.106.669432.
van Veldhuisen DJ, Genth-Zotz S, Brouwer J, et al: High- versus low-dose ACE inhibition in chronic heart failure: a double-blind, placebo-controlled study of imidapril. J Am Coll Cardiol. 1998, 32: 1811-1818. 10.1016/S0735-1097(98)00464-1.
Vanhoutte D, Schellings M, Pinto Y, Heymans S: Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006, 69: 604-613. 10.1016/j.cardiores.2005.10.002.
Herzog E, Gu A, Kohmoto T, Burkhoff D, Hochman JS: Early activation of metalloproteinases after experimental myocardial infarction occurs in infarct and non-infarct zones. Cardiovasc Pathol. 1998, 7: 307-312. 10.1016/S1054-8807(98)00008-8.
Peterson JT, Hallak H, Johnson L, et al: Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001, 103: 2303-2309. 10.1161/01.CIR.103.18.2303.
Spinale FG: Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002, 90: 520-530. 10.1161/01.RES.0000013290.12884.A3.
Webb CS, Bonnema DD, Ahmed SH: Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006, 114: 1020-1027. 10.1161/CIRCULATIONAHA.105.600353.
Orn S, Manhenke C, Squire IB, Ng L, Anand I, Dickstein K: Plasma MMP-2, MMP-9 and N-BNP in long-term survivors following complicated myocardial infarction: relation to cardiac magnetic resonance imaging measures of left ventricular structure and function. J Cardiac Fail. 2007, 13: 843-849. 10.1016/j.cardfail.2007.07.006.
Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB: Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007, 28: 711-718. 10.1093/eurheartj/ehm003.
Hudson MP, Armstrong PW, Ruzyllo W, et al: Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006, 48: 15-20. 10.1016/j.jacc.2006.02.055.
Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM: Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004, 10: 328-333. 10.1016/j.cardfail.2003.11.003.
Matsunaga T, Abe N, Kameda K, et al: Circulating level of gelatinase activity predicts ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol. 2005, 105: 203-208. 10.1016/j.ijcard.2005.01.011.
Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL: Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992, 267: 4583-4591.
Sato H, Takino T, Kinoshita T, et al: Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett. 1996, 385: 238-240. 10.1016/0014-5793(96)00389-4.
Kandalam V, Basu R, Abraham T, et al: TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res. 2010, 106: 796-808. 10.1161/CIRCRESAHA.109.209189.
Hojo Y, Ikeda U, Zhu Y, et al: Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol. 2000, 35: 968-973. 10.1016/S0735-1097(99)00632-4.
Heeschen C, Dimmeler S, Hamm CW, et al: Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003, 107: 524-530. 10.1161/01.CIR.0000048183.37648.1A.
de Boer RA, Yu L, van Veldhuisen DJ: Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010, 7: 1-8. 10.1007/s11897-010-0004-x.
Sharma UC, Pokharel S, van Brakel TJ: Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004, 110: 3121-3128. 10.1161/01.CIR.0000147181.65298.4D.
van Kimmenade RR, Januzzi JL, Ellinor PT, et al: Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006, 48: 1217-1224. 10.1016/j.jacc.2006.03.061.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/13/91/prepub
Acknowledgements
We thank the patients who enrolled in the study, their physicians, and the staff at the study sites for their important contributions; and Dr. Harmony Reynolds, Carole Russo, and Anna Yick, for assistance in the preparation of the manuscript.
Funding sources
OAT was supported by National Heart, Lung, and Blood Institute Awards U01HL062509 and U01HL062511. OAT Biomarker substudy was co-funded by grant of Institute of Cardiology 2.51/III/07.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MK – 1) have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; 2) have been involved in drafting the manuscript; and 3) have given final approval of the version to be published; VM, ZS, WR, JH, GO, KZ, SF, DL have made substantial contributions to conception and design, analysis and interpretation of data; 2) have been involved in revising the manuscript critically for important intellectual content; and 3) have given final approval of the version to be published; JK, JJ, MR, PC, MK, BBP, EZ, WJ, KJ, AR, GS 1) have made substantial contributions to acquisition of data; 2) have been involved in revising the manuscript critically for important intellectual content; and 3) have given final approval of the version to be published.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kruk, M., Menon, V., Kądziela, J. et al. Impact of percutaneous coronary intervention on biomarker levels in patients in the subacute phase following myocardial infarction: the Occluded Artery Trial (OAT) biomarker ancillary study. BMC Cardiovasc Disord 13, 91 (2013). https://doi.org/10.1186/1471-2261-13-91
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-13-91