Abstract
Background
Multiple randomized controlled trials (RCTs) have examined the cardiovascular effects of omega-3 fatty acids and have provided unexplained conflicting results. A meta-analysis of these RCTs to estimate efficacy and safety and potential sources of heterogeneity may be helpful.
Methods
The Cochrane library, MEDLINE, and EMBASE were systematically searched to identify all interventional trials of omega-3 fatty acids compared to placebo or usual diet in high-risk cardiovascular patients. The primary outcome was all-cause mortality and secondary outcomes were coronary restenosis following percutaneous coronary intervention and safety. Meta-analyses were carried out using Bayesian random-effects models, and heterogeneity was examined using meta-regression.
Results
A total of 29 RCTs (n = 35,144) met our inclusion criteria, with 25 reporting mortality and 14 reporting restenosis. Omega-3 fatty acids were not associated with a statistically significant decreased mortality (relative risk [RR] = 0.88, 95% Credible Interval [CrI] = 0.64, 1.03) or with restenosis prevention (RR = 0.89, 95% CrI = 0.72, 1.06), though the probability of some benefit remains high (0.93 and 0.90, respectively). However in meta-regressions, there was a >90% probability that larger studies and those with longer follow-up were associated with smaller benefits. No serious safety issues were identified.
Conclusions
Although not reaching conventional statistical significance, the evidence to date suggests that omega-3 fatty acids may result in a modest reduction in mortality and restenosis. However, caution must be exercised in interpreting these benefits as results were attenuated in higher quality studies, suggesting that bias may be at least partially responsible. Additional high quality studies are required to clarify the role of omega-3 fatty acid supplementation for the secondary prevention of cardiovascular disease.
Similar content being viewed by others
Background
Interest in the therapeutic value of fish oils began in the 1970's following the observation of a low incidence of cardiovascular disease in Greenland Inuits [1–3]. Subsequently, several studies identified eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), two major long chain n-3 polyunsaturated fatty acids (n-3 PUFAs), as the putative protective constituents [4–10]. The role of α-linolenic acid (ALA), a shorter chain omega-3, is still being debated [11]. Although the exact cardio-protective mechanisms of omega-3 fatty acids are unknown, it has been hypothesized that a reduction of arrhythmias, heart rate, ischemia/reperfusion-induced injury, serum triglyceride levels, inflammation, or improved endothelial function may be involved [12–17]. Multiple studies, reviews, and meta-analyses have been conducted in order to elucidate the effect of omega-3 fatty acids on cardiovascular outcomes, but controversy prevails with both positive [18] and negative conclusions [19, 20].
Omega-3 fatty acids are inexpensive compounds with an apparent favorable risk profile including a low propensity for drug interaction. Moreover, there have been several recent publications of randomized trials, and we therefore decided to perform an updated systematic review and meta-analysis of their cardiovascular efficacy. We have, unlike previous analyses [18, 19], restricted our systematic review to high risk patients, the group most likely to benefit from their use, and have explicitly investigated potential sources of heterogeneity between these trials.
Methods
Literature Search
We searched MEDLINE, the Cochrane Library, and EMBASE without language restrictions for original research articles, systematic reviews, and meta-analyses to identify all available literature on omega-3 fatty acids and cardiovascular disease published from January 1966 through September 2008. We combined search terms for omega-3 fatty acids ("omega-3 fatty acids" OR "fish oil" OR "marine oil" OR "dietary therapy") with those for mortality or cardiovascular disease ("mortality" OR "cardiovascular disease" OR "heart disease" OR "CAD" OR "MI" OR "UA" OR "coronary angiography" OR "coronary restenosis"). References of relevant articles were hand-searched for additional studies.
Inclusion criteria required each study to 1) be a comparative randomized trial involving human participants with an active treatment arm using omega-3 fatty acids with usual diet; 2) involve a high-risk population with known cardiovascular disease or diabetes; and 3) report at least 1 of the following 2 outcomes: total mortality or coronary artery restenosis following angioplasty. We excluded non-randomized studies, those involving children or animals, and studies in which the omega-3/fish supplement dosage was unspecified. We also excluded studies in which patients were randomized to dietary advice that included a non-quantifiable intervention of simply increasing fish consumption [21]. The Quality of Reporting of Meta-analyses of Randomized Controlled Trials (QUORUM) guidelines were followed throughout this meta-analysis [22] [See Additional file 1 - QUORUM checklist].
Data Extraction
One author performed the literature search, and data extraction was independently conducted by at least two individuals. The following information was extracted: publication details, timing of study, duration of follow-up, randomization method, blinding (of participants, investigators, and outcome assessors), omega-3 dosage, dropouts, mean age of participants, primary outcome (total mortality), secondary cardiovascular outcomes (restenosis, sudden death, cardiac death, non-fatal myocardial infarction (MI), congestive heart failure (CHF), arrhythmias, implantable cardioverter defibrillator (ICD) shocks, and stroke), the proportion of patients who discontinued treatment, side effects (gastro-intestinal (GI) side effects, bleeding, and malignancies), and adherence. Any disagreements in the collected data was resolved by consensus or, when necessary, upon consultation with a third reviewer. The authors of the original publications were contacted to obtain missing data and resolve ambiguities (n = 16), although we made no attempt to contact authors of articles published before 1995.
Quality assessment of the individual trials was performed using the Jadad scale [23] [See Additional file 2-Quality assessment of included trials].
Statistical Analysis
To estimate the risk of all-cause mortality, data were analyzed according to the intention-to-treat principal; the denominator was the number of participants randomized to each group, and the numerator was the number of deaths reported during the follow-up period. In our restenosis analysis, the denominator was the number of participants undergoing follow-up coronary angiography, and the numerator was those with restenosis. In most studies, restenosis was defined as the loss of luminal diameter of at least 50% [24–29]. One study defined it as a loss of 70% [30], and 2 studies defined coronary restenosis as at least 50% stenosis at follow-up [31, 32]. Three trials used multiple definitions, including loss of luminal diameter of at least 50% and at least 50% stenosis at follow-up [33–35]. The remaining two studies used unique restenosis definitions; one used a panel of blinded cardiologists to assess changes in progression and regression of CAD [36], and the other defined restenosis as lumens with greater than 20% obstruction [37]. Where possible, cardiovascular event and safety data were analyzed using an intention-to-treat approach. However, many restenosis studies presented data only for those who underwent follow-up coronary angiography.
For each study, we estimated the risk ratio (RR) comparing intervention and control groups. For studies with zero outcomes in either group, we added 0.5 to all cells of the 2-by-2 table. For the primary outcomes of mortality and main secondary analysis of restenosis, we fit meta-regression models to investigate if study-level covariates explained any of the heterogeneity in RRs across studies. We estimated the median and corresponding 95% credible interval (CrI), the Bayesian analogue for confidence intervals, for each coefficient in these models. In addition, we also estimated the probability that the coefficient was greater than 0. The covariates used in the meta-regression were median follow-up time (months), sample size, high dosage of the intervention, high quality (on the Jadad scale), high adherence rate, and high percentage of previous MI. Cut-offs for defining dichotomous covariates were determined by the median value across studies. For the all-cause mortality meta-analysis, subgroups were defined by sample size (>322 patients), study quality (>3), dosage of omega-3 (>3.3 g/day), adherence (>84%), and history of previous MI (>50%). For the restenosis meta-analysis, subgroups were defined by study quality (>3), sample size (>233 patients), dosage of omega-3 (>5.04 g/day), adherence (>85%), and previous MI (>48%).
We carried out meta-analyses to pool RRs across all studies [38]. Separate models were created for each of the primary and secondary outcomes and for safety data. When there was greater than 90% probability that a covariate was associated with the relative risk (i.e., >90% probability that the meta-regression coefficient was different from 0), we carried out separate meta-analyses within subgroups defined by the covariate. Funnel plots were employed to assess publication bias.
All analyses were carried out using WinBUGS and R software programs. Low information prior distributions (mean 0 and a standard deviation of 100) were used for all parameters. The between-study standard deviation in the log (RR) was assumed to follow a uniform distribution over the range from 0 to 5.
Results
Literature Search
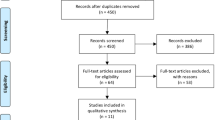
Our literature search identified 9,258 titles, of which 558 articles were considered potentially relevant (Figure 1). The full texts of these articles were retrieved, and 27 articles fulfilled our inclusion criteria [24–37, 39–51]. One trial [51] was then excluded due to concerns regarding the authenticity of its data [52–54]. Three additional trials were identified through our hand-search of relevant studies [55–57], resulting in a total of 29 included trials. These trials involved a total of 35,144 patients. There were 25 articles that reported mortality [24–26, 29, 31, 33–37, 39–50, 55–57] and 14 that examined coronary restenosis [25–37, 42]. The trials reporting mortality randomized 34,501 patients (17,276 omega-3 patients and 17,190 controls), and the restenosis trials randomized 3,553 patients to omega-3 fatty acids (n = 1,817) or control (n = 1,736).
Study and Patient Characteristics
Although performed in a multitude of different countries, the trials were fairly homogeneous with respect to patient and study characteristics [See Additional file 3 - study and patient characteristics]. All mortality trials were conducted in high-risk cardiovascular patients: 4 were conducted in post-MI patients [21, 24, 43, 44], 3 involved patients with ICDs [47–49], and 1 involved diabetic patients [41]. The remaining 16 trials consisted of a mixture of other high-risk cardiovascular patients. Most trials were conducted in middle aged or older patients, and the majority of patients were men. Trials were generally double-blind, with either placebo or inactive oil as control, although some were open-label with control being usual diet [28, 44]. Thirteen mortality trials and 2 restenosis trials had follow-up ≥ 12 months. In the mortality trials, omega-3 was provided in the form of EPA, DHA, DPA, or ALA, with dosages ranging from 0.9 g/day to 6.9 g/day. In the restenosis trials, omega-3 consisted of EPA or DHA, with daily dosages ranging from 3 g/day to 6.9 g/day. Follow-up varied from 1 to 55 months with a median 6 months for restenosis studies and 12 months for mortality studies.
All-Cause Mortality
A total of 3,867 deaths (1,885 in omega-3 patients and 1,982 in controls) occurred in these trials. When data were pooled across all studies, omega-3 fatty acids were not associated with a reduction in all cause-mortality (RR = 0.88, 95% CrI = 0.64, 1.03), though the probability of some benefit (RR<1) remains high (0.93). The majority of the studies were inconclusive but one individual study with a follow-up greater than 12 months did show a statistically significant beneficial effect of omega-3 fatty acids (Figures 2, 3).
The meta-regression models suggested with a greater than 90% probability that larger studies and those with longer follow-up were associated with decreasing health benefits (Table 1). Specifically, there was a 97% probability that the effect of omega-3 fatty acids was smaller in the 12 larger trials (RR = 0.95, 95% CrI = 0.76, 1.13) than in the 13 trials with small sample sizes (RR = 0.47, 95% CrI = 0.18, 0.83). Similarly, there was a 99% probability that those with follow-up greater than 12 months (RR = 0.91, 95% CrI = 0.57, 1.12) had attenuated benefits compared with those with follow-up ≤ 12 months (RR = 0.72, 95% CrI = 0.38, 1.08) (Figures 2, 3 and 4). Dosage, study quality, adherence, and history of previous MI did not appear to influence the results.
We also carried out a sensitivity analysis by excluding the sole study using ALA [24], which did not change the overall results.
Coronary Restenosis
In 14 studies that assessed omega-3 fatty acids following a percutaneous coronary intervention (PCI), these agents were not statistically associated with the risk of restenosis (RR = 0.89, 95% CrI = 0.72, 1.05)(Figure 5) but again there was a moderate probability of some benefit (probability RR < 1 = 0.90). Subgroup analysis suggested that this result was unaltered by dosage, follow-up, or adherence (Table 1) but there was a 94% probability that the effect of omega-3 fatty acids on restenosis was attenuated in larger RCTs (RR = 0.94, 95% CrI = 0.66, 1.26) compared with smaller ones (RR = 0.82, 95% CrI = 0.56, 1.17) and a 89% probability that higher quality trials also exhibited attenuated results.
Effect of omega-3 fatty acids on coronary restenosis. Arrows indicate that the lower limit of the credible interval for the relative risk was less than 0.2 or the upper limit exceeded 5.0. Although 3,553 patients were randomized to omega-3 fatty acids (n = 1,817) or control (n = 1,736), only 3,004 patients (1,539 and 1,465, respectively) were included in the analyses. Patients who did not undergo follow-up angiogram were generally excluded from the restenosis analyses.
Other Cardiovascular Events
Although the point estimates suggest that omega-3 fatty acids may reduce other cardiovascular events, including cardiac death, sudden death, non-fatal MI, CHF, arrhythmias, and ICD shocks, the wide CrIs prevent definitive conclusions from being drawn [See Additional file 4 - secondary cardiovascular outcomes].
Safety
Few studies reported side effect and safety data (Table 2). Patients randomized to omega-3 fatty acids did have an increased risk of GI side-effects (RR = 1.29, 95% CrI = 1.02, 1.61). However, the risk of treatment discontinuation was similar between groups (RR = 1.23, 95% CrI = 0.87, 1.68), suggesting that these side-effects were relatively minor. There was no increased risk of malignancies (RR = 1.02, 95% CrI = 0.73, 1.25) or bleeding (RR = 0.85, 95% CrI = 0.52, 1.27), but wide 95% CrI again prevent definitive conclusions from being drawn regarding these outcomes.
Funnel plots did not provide any clear indication of publication bias (data not shown).
Discussion
Our systematic review and meta-analysis assessed the effect of omega-3 fatty acids on all-cause mortality and coronary artery restenosis following PCI among high-risk cardiovascular patients. Omega-3 fatty acids were not associated with a statistically significant reduction in all-cause mortality or restenosis but the probability of a modest benefit remains considerable. Omega-3 fatty acids also had generally favorable effects on other cardiovascular outcomes, but definitive conclusions are not forthcoming due to the small number of studies that reported these outcomes. Unfortunately, the majority of trials did not systematically record adherence and side effects. Nevertheless, the available data suggest a favorable side-effect profile.
Our meta-regression identified some important sources of heterogeneity among mortality trials, including trial size and follow-up time. Specifically, larger and longer trials had smaller mortality benefits, suggesting that the overall observed benefit may be at least partially inflated due to bias. Similarly, restenosis benefits were smaller in larger, better quality trials. These findings temper our enthusiasm for this intervention despite a relatively favorable risk profile. Definitive results about the efficacy and safety of omega-3 fatty acid supplementation will benefit from the results of currently ongoing clinical trials, including ORIGIN [58] and ASCEND [59].
The effect of omega-3 fatty acids has been examined in previous systematic reviews and meta-analyses. However, these earlier meta-analyses [19, 60, 61] were not limited to high-risk cardiovascular patients, included a scientifically questionable study [51], did not have access to the most recently published large studies of 15,000 high-risk cardiovascular patients [47–50, 55, 57], and importantly, did not explicitly investigate potential sources of heterogeneity which permits a more nuanced interpretation of the totality of evidence. Our results are similar to previous focused meta-analysis examining restenosis [62] and ICD shocks [61, 63]. However, our credible intervals are slightly wider as our Bayesian methods account for uncertainty in the between-study variability.
Strengths and Limitations
Our systematic review and meta-analysis has a number of strengths. First, it provides a complete and comprehensive review of the current state of the omega-3 fatty acid literature. In several cases, the published data of this systematic review have been complimented by additional data furnished by the principal investigators of the original studies. Second, our Bayesian models, unlike their frequentist counterparts, allow for the calculation of probabilities and therefore have a more intuitive and informative interpretation. Third, our systematic review and meta-analysis was conducted according to a pre-specified protocol, including pre-specified subgroup analyses, and without language restrictions. Fourth, we have addressed not only the efficacy but also the safety of omega-3 fatty acids. Finally, although previous reports have discussed the role of heterogeneity in the literature [64], we examined the sources of heterogeneity analytically. Consequently, we have provided a thorough and methodologically rigorous synthesis of the available evidence thereby facilitating informed decision making.
Nevertheless, our systematic review and meta-analysis does have potential limitations. First, as is true with all systematic review and meta-analyses, our study may be affected by publication bias, although we did not find evidence of its occurrence. Second, there was some heterogeneity in study design, including in dosage of omega-3 fatty acid used and patient populations. In particular, there is much uncertainty regarding the potency and purity of over-the-counter formulations while the proprietary formulation is both expensive and has been infrequently used in the clinical trials. Moreover, the control groups were exposed to varying amounts of fish oils according to national dietary habits, and we could not account for this variability. Our random-effects models attempt to account for between-study variability, and the effects of this heterogeneity were examined in our meta-regression models. Third, safety data were not reported in all studies. Fourth, due to the fish odor of omega-3 fatty acid supplements, complete blinding of fish oil studies may not be feasible. This imperfect blinding was not considered in our quality assessment. Fifth, most restenosis studies only presented data among those who completed their follow-up angiogram. Consequently, restenosis data were generally analyzed using a modified intention-to-treat, which may result in biased results. Finally, due to the lack of individual-level data, we were not able to estimate the change in risk of mortality or cardiovascular outcomes over time. We therefore assumed that the risk of the outcome remained the same across the duration of each study and that any censoring was random. Availability of individual-level data would also have allowed us to estimate the effect of patient-level covariates and to examine which subgroups may derive the greatest benefit from the use of these agents.
Conclusions
This meta-analysis demonstrates that omega-3 fatty acid supplementation may modestly reduce all-cause mortality and restenosis when used as secondary prevention. However, the mortality benefit was attenuated in larger RCTs and those with longer follow-up, and the restenosis benefits were similarly reduced in larger RCTs. These results suggest that bias may at least partially explain the observed benefit. Also, many studies had incomplete information on other cardiac endpoints and adverse events. Further ongoing studies with sufficient sample size, standardized dosing, and adequate follow-up duration are required to clarify the role of omega-3 fatty acid supplementation for the secondary prevention of cardiovascular disease.
References
Bang HO, Dyerberg J, Hjoorne N: The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976, 200: 69-73.
Bang HO, Dyerberg J: Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand. 1972, 192: 85-94.
Bang HO, Dyerberg J: Lipid metabolism and ischemic heart disease in Greenland Eskimos. Advances in Nutrition Research. Edited by: Draper H. 1980, New York, N.Y.: Plenum Press, 1-22.
Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB: Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997, 336: 1046-1053. 10.1056/NEJM199704103361502.
Din JN, Newby DE, Flapan AD: Omega 3 fatty acids and cardiovascular disease--fishing for a natural treatment. BMJ. 2004, 328: 30-35. 10.1136/bmj.328.7430.30.
Dolecek TA, Granditis G: Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet. 1991, 66: 205-216.
Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS: Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003, 107: 1372-1377. 10.1161/01.CIR.0000055315.79177.16.
Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB: Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005, 111: 157-164. 10.1161/01.CIR.0000152099.87287.83.
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J: n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006, 84: 5-17.
Yuan JM, Ross RK, Gao YT, Yu MC: Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001, 154: 809-816. 10.1093/aje/154.9.809.
Nettleton JA: Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc. 1991, 91: 331-337.
Xiao YF, Sigg DC, Leaf A: The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005, 206: 141-154. 10.1007/s00232-005-0786-z.
Engelbrecht AM, Engelbrecht P, Genade S, Niesler C, Page C, Smuts M, Lochner A: Long-chain polyunsaturated fatty acids protect the heart against ischemia/reperfusion-induced injury via a MAPK dependent pathway. J Mol Cell Cardiol. 2005, 39: 940-954. 10.1016/j.yjmcc.2005.08.004.
Harrison N, Abhyankar B: The mechanism of action of omega-3 fatty acids in secondary prevention post-myocardial infarction. Curr Med Res Opin. 2005, 21: 95-100. 10.1185/030079904X17956.
Geelen A, Brouwer IA, Zock PL, Katan MB: Antiarrhythmic effects of n-3 fatty acids: evidence from human studies. Curr Opin Lipidol. 2004, 15: 25-30. 10.1097/00041433-200402000-00006.
Kris-Etherton PM, Harris WS, Appel LJ: Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002, 106: 2747-2757. 10.1161/01.CIR.0000038493.65177.94.
Connor WE: Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000, 71: 171S-175S.
Bucher HC, Hengstler P, Schindler C, Meier G: N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002, 112: 298-304. 10.1016/S0002-9343(01)01114-7.
Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, et al: Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006, 332: 752-760. 10.1136/bmj.38755.366331.2F.
Yzebe D, Lievre M: Fish oils in the care of coronary heart disease patients: a meta-analysis of randomized controlled trials. Fundam Clin Pharmacol. 2004, 18: 581-592. 10.1111/j.1472-8206.2004.00268.x.
Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM: Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989, 2: 757-761. 10.1016/S0140-6736(89)90828-3.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999, 354: 1896-1900. 10.1016/S0140-6736(99)04149-5.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996, 17: 1-12. 10.1016/0197-2456(95)00134-4.
Borchgrevink CF, Skaga E, Berg KJ, Skjaeggestad O: Absence of prophylactic effect of linolenic acid in patients with coronary heart-disease. Lancet. 1966, 2: 187-189. 10.1016/S0140-6736(66)92474-3.
Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weiner BH, Slack JD, Kellett MA, Raizner AE, et al: Do fish oils prevent restenosis after coronary angioplasty?. Circulation. 1994, 90: 2248-2257.
Cairns JA, Gill J, Morton B, Roberts R, Gent M, Hirsh J, Holder D, Finnie K, Marquis JF, Naqvi S, et al: Fish oils and low-molecular-weight heparin for the reduction of restenosis after percutaneous transluminal coronary angioplasty. The EMPAR Study. Circulation. 1996, 94: 1553-1560.
Kaul U, Sanghvi S, Bahl VK, Dev V, Wasir HS: Fish oil supplements for prevention of restenosis after coronary angioplasty. Int J Cardiol. 1992, 35: 87-93. 10.1016/0167-5273(92)90059-C.
Bellamy CM, Schofield PM, Faragher EB, Ramsdale DR: Can supplementation of diet with omega-3 polyunsaturated fatty acids reduce coronary angioplasty restenosis rate?. Eur Heart J. 1992, 13: 1626-1631.
Grigg LE, Kay TW, Valentine PA, Larkins R, Flower DJ, Manolas EG, O'Dea K, Sinclair AJ, Hopper JL, Hunt D: Determinants of restenosis and lack of effect of dietary supplementation with eicosapentaenoic acid on the incidence of coronary artery restenosis after angioplasty. J Am Coll Cardiol. 1989, 13: 665-672. 10.1016/0735-1097(89)90609-8.
Reis GJ, Boucher TM, Sipperly ME, Silverman DI, McCabe CH, Baim DS, Sacks FM, Grossman W, Pasternak RC: Randomised trial of fish oil for prevention of restenosis after coronary angioplasty. Lancet. 1989, 2: 177-181. 10.1016/S0140-6736(89)90370-X.
Dehmer GJ, Popma JJ, van den Berg EK, Eichhorn EJ, Prewitt JB, Campbell WB, Jennings L, Willerson JT, Schmitz JM: Reduction in the rate of early restenosis after coronary angioplasty by a diet supplemented with n-3 fatty acids. N Engl J Med. 1988, 319: 733-740.
Milner MR, Gallino RA, Leffingwell A, Pichard AD, Brooks-Robinson S, Rosenberg J, Little T, Lindsay J: Usefulness of fish oil supplements in preventing clinical evidence of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1989, 64: 294-299. 10.1016/0002-9149(89)90522-5.
Bairati I, Roy L, Meyer F: Double-blind, randomized, controlled trial of fish oil supplements in prevention of recurrence of stenosis after coronary angioplasty. Circulation. 1992, 85: 950-956.
Franzen D, Schannwell M, Oette K, Hopp HW: A prospective, randomized, and double-blind trial on the effect of fish oil on the incidence of restenosis following PTCA. Cathet Cardiovasc Diagn. 1993, 28: 301-310. 10.1002/ccd.1810280407.
Maresta A, Balduccelli M, Varani E, Marzilli M, Galli C, Heiman F, Lavezzari M, Stragliotto E, De Caterina R: Prevention of postcoronary angioplasty restenosis by omega-3 fatty acids: main results of the Esapent for Prevention of Restenosis ITalian Study (ESPRIT). Am Heart J. 2002, 143: E5-10.1067/mhj.2002.121805.
von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H: The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999, 130: 554-562.
Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC: Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP Research Group. J Am Coll Cardiol. 1995, 25: 1492-1498. 10.1016/0735-1097(95)00095-L.
Warn DE, Thompson SG, Spiegelhalter DJ: Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2002, 21: 1601-1623. 10.1002/sim.1189.
Nye ER, Ablett MB, Robertson MC, Ilsley CD, Sutherland WH: Effect of eicosapentaenoic acid on restenosis rate, clinical course and blood lipids in patients after percutaneous transluminal coronary angioplasty. Aust N Z J Med. 1990, 20: 549-552.
Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M: Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996, 77: 31-36. 10.1016/S0002-9149(97)89130-8.
Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH: Fish oil in diabetic nephropathy. Diabetes Care. 1996, 19: 1214-1219. 10.2337/diacare.19.11.1214.
Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H: N-3 fatty acids do not prevent restenosis after coronary angioplasty: results from the CART study. Coronary Angioplasty Restenosis Trial. J Am Coll Cardiol. 1999, 33: 1619-1626. 10.1016/S0735-1097(99)00054-6.
Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L: Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001, 74: 50-56.
Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, et al: Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002, 105: 1897-1903. 10.1161/01.CIR.0000014682.14181.F2.
Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC: Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003, 57: 193-200. 10.1038/sj.ejcn.1601539.
Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M: N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005, 45: 1723-1728. 10.1016/j.jacc.2005.02.079.
Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D: Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005, 112: 2762-2768. 10.1161/CIRCULATIONAHA.105.549527.
Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, McClelland J, Cook J, MacMurdy K, Swenson R, et al: Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005, 293: 2884-2891. 10.1001/jama.293.23.2884.
Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, et al: Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006, 295: 2613-2619. 10.1001/jama.295.22.2613.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007, 369: 1090-1098. 10.1016/S0140-6736(07)60527-3.
Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M: Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival--4. Cardiovasc Drugs Ther. 1997, 11: 485-491. 10.1023/A:1007757724505.
Horton R: Expression of concern: Indo-Mediterranean Diet Heart Study. Lancet. 2005, 366: 354-356. 10.1016/S0140-6736(05)67006-7.
Mann J: The Indo-Mediterranean diet revisited. Lancet. 2005, 366: 353-354. 10.1016/S0140-6736(05)67005-5.
White C: Suspected research fraud: difficulties of getting at the truth. BMJ. 2005, 331: 281-288. 10.1136/bmj.331.7511.281.
GISSI-HF Investigators: Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008, 372: 1223-1230. 10.1016/S0140-6736(08)61239-8.
Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, France M: An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001, 85: 544-548. 10.1136/heart.85.5.544.
Randomized Trial of Omega - 3 Fatty Acids on Top of Modern Therapy after Acute Myocardial Infarction: The OMEGA-Trial. [http://assets.cardiosource.com/OMEGA_Senges.ppt]
Origin Trial Investigators, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J: Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J. 2008, 155: 26-32.
ASCEND: A Study of Cardiovascular Events iN Diabetes. [http://www.ctsu.ox.ac.uk/ascend/]
Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey SG, et al: Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev. 2004, CD003177-
Leon H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki RT: Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008, 337: a2931-10.1136/bmj.a2931.
Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J: Effects of omega-3 fatty acids on coronary restenosis, intima-media thickness, and exercise tolerance: a systematic review. Atherosclerosis. 2006, 184: 237-246. 10.1016/j.atherosclerosis.2005.06.042.
Jenkins DJ, Josse AR, Beyene J, Dorian P, Burr ML, LaBelle R, Kendall CW, Cunnane SC: Fish-oil supplementation in patients with implantable cardioverter defibrillators: a meta-analysis. CMAJ. 2008, 178: 157-164.
Jenkins DJ, Josse AR, Dorian P, Burr ML, LaBelle TR, Kendall CW, Cunnane SC: Heterogeneity in randomized controlled trials of long chain (fish) omega-3 fatty acids in restenosis, secondary prevention and ventricular arrhythmias. J Am Coll Nutr. 2008, 27: 367-378.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/10/24/prepub
Acknowledgements
We would like to thank Genevieve Gariepy MSc for her help with data extraction. Drs. Dendukuri and Brophy receive financial support from les Fonds de la Recherche en Santé du Québec. Dr. Filion received financial support from the Faculty of Medicine of McGill University, the Research Institute of the McGill University Health Centre, and the Department of Medicine of the McGill University Health Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JMB conceived of the study idea, and FE and JMB contributed to the study design. FE conducted the literature review. FE, MB, and KBF performed the data extraction and JMB, ND, and KBF were involved in consensus agreements concerning data discrepancies. FE and KBF drafted the manuscript. ND designed and ND and IS conducted the statistical analyses. All authors were involved in revising the article for important intellectual content, interpreting the data, and approved the final version to be published.
Kristian B Filion, Fouad El Khoury contributed equally to this work.
Electronic supplementary material
12872_2010_301_MOESM1_ESM.PDF
Additional file 1: QUORUM Checklist. Checklist detailing where the required elements of a thorough meta-analysis, as specified by the Quality of Reporting of Meta-Analyses (QUOROM) guidelines, can be located within the manuscript. (PDF 19 KB)
12872_2010_301_MOESM2_ESM.PDF
Additional file 2: Quality Assessment of Included Trials. Quality assessment of randomized controlled trials examining the effect of omega-3 fatty acids on all-cause mortality and restenosis using the Jadad scale. (PDF 11 KB)
12872_2010_301_MOESM3_ESM.PDF
Additional file 3: Study and Patient Characteristics. Study and patient characteristics of trials examining the effect of omega-3 fatty acids on all-cause mortality and coronary restenosis. (PDF 40 KB)
12872_2010_301_MOESM4_ESM.PDF
Additional file 4: Secondary Cardiovascular Outcomes. Randomized controlled trials reporting secondary cardiovascular outcomes, including arrhythmias, cardiac death, congestive heart failure, implantable cardioverter defibrillator shocks, non-fatal myocardial infarction, stroke, and sudden death. (PDF 42 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Filion, K.B., El Khoury, F., Bielinski, M. et al. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 10, 24 (2010). https://doi.org/10.1186/1471-2261-10-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-10-24