Abstract
Background
Epigenetic factors such as DNA methylation and histone modifications regulate a wide range of processes in plant development. Cytosine methylation and demethylation exist in a dynamic balance and have been associated with gene silencing or activation, respectively. In Arabidopsis, cytosine demethylation is achieved by specific DNA glycosylases, including AtDME (DEMETER) and AtROS1 (REPRESSOR OF SILENCING1), which have been shown to play important roles in seed development. Nevertheless, studies on monocot DNA glycosylases are limited. Here we present the study of a DME homologue from barley (HvDME), an agronomically important cereal crop, during seed development and in response to conditions of drought.
Results
An HvDME gene, identified in GenBank, was found to encode a protein with all the characteristic modules of DME-family DNA glycosylase proteins. Phylogenetic analysis revealed a high degree of homology to other monocot DME glycosylases, and sequence divergence from the ROS1, DML2 and DML3 orthologues. The HvDME gene contains the 5′ and 3′ Long Terminal Repeats (LTR) of a Copia retrotransposon element within the 3′ downstream region. HvDME transcripts were shown to be present both in vegetative and reproductive tissues and accumulated differentially in different seed developmental stages and in two different cultivars with varying seed size. Additionally, remarkable induction of HvDME was evidenced in response to drought treatment in a drought-tolerant barley cultivar. Moreover, variable degrees of DNA methylation in specific regions of the HvDME promoter and gene body were detected in two different cultivars.
Conclusion
A gene encoding a DNA glycosylase closely related to cereal DME glycosylases was characterized in barley. Expression analysis during seed development and under dehydration conditions suggested a role for HvDME in endosperm development, seed maturation, and in response to drought. Furthermore, differential DNA methylation patterns within the gene in two different cultivars suggested epigenetic regulation of HvDME. The study of a barley DME gene will contribute to our understanding of epigenetic mechanisms operating during seed development and stress response in agronomically important cereal crops.
Similar content being viewed by others
Background
Epigenetic regulation during plant development and in response to environmental conditions is attained by DNA methylation, histone modifications, small interfering RNAs (siRNAs) and long non-coding RNAs (Long ncRNAs), leading to changes in chromatin structure. Open and closed chromatin states are associated with gene activation and gene silencing, respectively, and govern proper onset of gene expression programmes during different developmental processes and in response to changing environmental conditions [1–3].
A dynamic interplay between DNA methylation and demethylation accomplished through specific enzymes, is critical for proper cellular regulation during plant development [4, 5]. Even though DNA methylation is a relatively stable epigenetic mark, it is subject to passive or active demethylation during the development of an organism [6]. Passive demethylation can occur when methylated cytosines are replaced by non-modified cytosines during DNA replication. Active DNA demethylation is achieved by specific DNA glycosylases through the base-excision-repair (BER) pathway by hydrolysis of the N-glycosidic bond between the ribose and the base [5, 7, 8]. Recent studies in animals have implicated another mechanism for DNA demethylation initiating with the hydroxylation of 5 methyl-cytosine by TET1 methylcytosine dioxygenase followed by the BER pathway that leads to DNA demethylation [9–11]. Such a mechanism is not found in plants, thus far. In plants demethylation by the BER pathway is operated by DNA glycosylases that excise 5 methylcytosine from the DNA sugar backbone and cleave the backbone at the abasic site [6]. In Arabidopsis, four such DNA glycosylase have been described, DEMETER (DME), REPRESSOR OF SILENCING (ROS1), DEMETER LIKE2 (DML2) and DEMETER LIKE3 (DML3), also called DME-family DNA glycosylases. AtDME and AtROS1 are bifunctional DNA glycosylases/AP-lyases that excise 5-methylcytosine and subsequently cleave the ribose-base phosphodiester bond whereas the resulting gap is filled by a DNA polymerase and the repair process is completed by a DNA ligase [12–15].
DME-family DNA glycosylases (DME, ROS1, DML2, DML3) have both common and different structural features as compared to typical DNA glycosylases. The glycosylase domain of DME-family proteins harbours the conserved helix–hairpin–helix (HhH) motif and a glycine/proline-rich region followed by a conserved aspartic acid (GPD) also found in human 8-oxoguanine DNA glycosylase (hOGG1), Escherichia coli adenine DNA glycosylase (MutY), and endonuclease III (Endo III) [16–18]. Moreover, similar to MutY and Endo III, DME-family DNA glycosylases contain four conserved cysteine residues flanking the DNA glycosylase domain that may function to hold a [4Fe-4S] cluster in place. Unlike other members of the HhH DNA glycosylase superfamily, DME-family members contain two additional conserved domains (domain A and domain B) flanking the central glycosylase domain [18]. Mutagenesis analysis of AtDME has revealed that the conserved DNA glycosylase domain and flanking domains A, and B, are necessary and sufficient for DME enzymatic activity [18].
Initial reports had implicated AtDME in demethylating genes of the female gametophyte involved in endosperm development whereas AtROS1, AtDML2 and AtDML3 were found expressed in vegetative tissues targeting transposons, repetitive elements and small RNA-generating loci [19–21]. AtDME was originally characterized as an epigenetic regulator required for maternal allelelic expression of the MEDEA (MEA) gene, encoding a H3K27 methylatransferase, in the central cell and endosperm [12, 22, 23]. In Arabidopsis, proper embryo and endosperm development depends on the expression of the maternal allele of the Polycomb group Polycomb Repressive complex (PRC2) encoding genes: MEA, FERTILIZATION INDEPENDENT ENDOSPERM (FIE), and FERTILIZATION INDEPENDENT SEED2 (FIS2) [22–24]. MEA, FIE and FIS2 play a role in preventing the onset of central cell proliferation by repressing the expression of target genes, among them the Type-I MADS-box gene PHERES1 (PHE1) [23, 25, 26].
The first reports on global DNA methylation profiling of endosperm and embryo genomes demonstrated wide-spread reduction of DNA methylation in the endosperm, particularly at regions corresponding to TE and small RNAs [27, 28]. These were largely due to AtDME action in the central cell, the progenitor of the endosperm, which develops after fusion of the central cell with one of the sperm cells of the pollen. Though AtDME action is restricted to the central cell, global demethylation persists in the developing endosperm post-fertilization. It was proposed that imprinted genes are not specific sequences targeted for demethylation but rather the result of a universal process carried out primarily through the action of DME that reconfigures DNA methylation of the entire maternal genome in the endosperm [28]. Recently it was demonstrated that AtDME is responsible for all of the active DNA demethylation taking place in the central cell and it preferentially targets small, AT-rich, and nucleosome-depleted euchromatic transposable elements [29]. AtDME also demethylates similar sequences in the vegetative cell of the male gametophyte and suppression of AtDME in the vegetative cell causes reduced small RNA–directed DNA methylation of transposons in its companion sperm cell [29–31].
Unlike the extensive investigations in Arabidopsis, studies on DNA glycosylases in monocots are limited. DNA demethylation was shown to result in the activation of a wide range of protein coding genes as well as transposable elements in rice endosperm. Interestingly, knockout mutants of a rice ROS1 gene which demethylates retrotransposn Tos17 led to wrinkled seeds as compared to wild type plants, suggesting that rice ROS1 is involved in seed development [32]. Likewise, a null mutation of rice ROS1a leads to abnormal early endosperm development and nonviable seed [33]. Finally, extensive efforts to understand and treat gluten-intolerance and celiac disease in population groups have led to the isolation and genetic engineering of wheat and barley DME homologues (among other genes) [34–39]. Importantly, downregulation of wheat DME resulted in decreased expression of endosperm prolamins, a powerful immunoreactant in celiac disease patients [39].
During the past several years our group has studied genes encoding epigenetic regulators and their putative targets, during seed development and in response to stress in barley, an agronomically important cereal crop [40–48]. In this study we have extended our exploration of epigenetic regulation in barley, by investigating the possible role of an HvDME gene, in seed development and in response to drought stress in different barley cultivars.
Methods
Plant material
Seeds for commercial barley cultivars, Caresse, Kos, Ippolytos and Demetra differing in seed size, weight and drought tolerance, were kindly provided by the Cereal Institute at the National Agricultural Research Foundation of Greece (http://www.cerealinstitute.gr) and were the source of total RNA and genomic DNA. For Caresse the weight of 1000 grains is 50–55 gr and 98% of seeds have diameter longer than 2.5 mm, for Kos the weight of 1000 grains is 36–40 gr and 75% of seeds have diameter longer than 2.5 mm, whereas for Ippolytos, seeds weight 25–31 gr per 1000 grains and only 35–45% of seeds have diameter longer than 2.5 mm. Caresse has facultative growth and is characterized by intermediate tolerance to abiotic stress/drought whereas Demetra is also a facultative type cultivar, drought-tolerant and very adaptable to a variety of soil-climatic conditions. The weight per 1,000 grains is 38–44 g and 70% of seeds have diameter longer than 2.5 mm (http://www.cerealinstitute.gr).
Drought experiment
An open hydroponic-type arrangement was used for the experimental setup consisting of 6 pots from each cultivar (Caresse and Demetra), which were constantly irrigated with tap water (pH 6–7). Three seedlings were grown inside each pot. Seedlings were allowed to grow for up to 7 days, at which time 3 pots were removed from the hydroponic setup and placed into separate dry plates. These were water-withheld for a total of 10 days. The other 3 pots were used as controls and were kept in well-watered conditions. Aerial parts from each pot were pulled together so each biological repeat is represented by 9 plants. Two biological replicates were conducted. The aerial parts of seedlings were harvested the 3rd and 10th day and were stored in −80°C until further use.
RNA isolation and first strand cDNA synthesis
Total RNA was isolated from roots, meristems, seedlings, leaves, flowers before fertilization (immature flowers), seeds 1–3, 3–5, 5–10, 10–15 days after fertilization (DAF), using TRI REAGENT 3 (SIGMA) according to the instructions of the manufacturer. First strand cDNA synthesis was performed using 1.0 μg total RNA, 0.5 μg 3′ RACE Adapter primer, 5′-5 GGCCACGCGTCGACTAGTAC (T)17-3′ (Invitrogen), 1 mM dNTPs and 200U of Superscript II (Invitrogen) in 20 μL total volume, according to the specifications of the manufacturer.
Protein sequence analysis
Multiple alignment was created with ClustalW. The phylogenetic tree was calculated using MEGA 5.0 software [49] by the Neighbor-Joining Method with p-distance correction [50]. Bootstrap values were obtained from 1000 bootstrap replicates. The 3D-structures were predicted using swiss-model (http://swissmodel.expasy.org/) and visualized with FirstGlance in Jmol (http://oca.weizmann.ac.il/oca-docs/fgij/slides.htm). Accession numbers of sequences used for alignments and phylogenetic analysis are indicated in Additional file 1: Table S1.
Genomic organization
The DME genomic sequences of Brachypodium distachyon (Bradi4g08870.1), Oryza sativa (01 g11900.1) and Zea mays (GRMZM2G123587) were downloaded from the Phytozome database (http://www.phytozome.net/). The sequence of HvDME was obtained from GenBank (BAC 273i4, accession number FM164415.1). Genomic organization of exons and introns was obtained using the mRNA-to-genomic alignment Spidey tool, in NCBI (http://www.ncbi.nlm.nih.gov/spidey). Detection of retroelements was performed with the MASiVE and LTRharvester tools (http://tools.bat.ina.certh.gr/masive/) (http://tools.bat.ina.certh.gr/ltrharvester/) and homology was visualized with Circoletto (http://tools.bat.ina.certh.gr/circoletto/). Statistically significant prediction for CpG islands was performed using the online predictor, which is part of the sequence manipulation suite at http://www.bioinformatics.org/SMS/index.html.
Expression analysis of HvDME in tissues and under drought
Qualitative RT-PCR and quantitative real-time RT-PCR was performed with cDNA synthesized from 1 μg of total RNA from roots, stems, meristems, leaves, immature flowers, seeds 1–3 DAF, 3–5 DAF, 5–10 DAF, 10–15 DAF and aerial parts of seedlings after drought treatment. For real-time PCR, each sample reaction was set up in a PCR reaction mix (20 μl) containing 5 μl of the 1:50 diluted cDNA, 0.25 μM of each primer and 1× Platinum SYBR Green qPCR Supermix-UDG (Invitrogen, Paisley, UK) and using the Corbett Rotor Gene 6000. Each reaction was performed in triplicate. General thermocycler conditions were 50°C for 2 min, 95°C for 2 min, then 42 cycles [denaturing at 95°C for 20 sec, annealing at 56°C for 25 sec, extension at 72°C for 25 sec], then 72°C for 10 min. To identify the PCR products a melting curve was performed from 65°C to 95°C with observations every 0.2°C and a 10-s hold between observations. Relative quantification was performed using actin as the reference gene and HvActinF/HvActinR as primers. Primers used in expression analysis correspond to non-conserved regions and are shown in Additional file 2: Table S2.
DNA methylation assays
Genomic DNA was prepared from control and drought-treated seedlings (Caresse and Demetra) with Qiagen columns following the protocol of the manufacturer (Qiagen Plant genomic DNA kit). Cytosine DNA methylation was analyzed by restricting 1 μg of genomic DNA from each sample with the methylation-dependent enzyme McrBC (NEB Biolabs), according to the manufacturer’s instructions, and PCR-amplifying equal quantities of McrBC-treated and untreated samples. Primers used are shown in Additional file 2: Table S2.
Results
HvDME protein sequence analysis
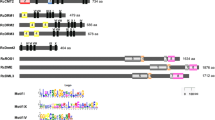
A gene sequence with accession number FM164415.1 corresponding to a barley HvDME gene from the cultivar Morex was retrieved from GenBank. This gene sequence had been deposited, annotated and described in GenBank (Langen, G., Pang, J., Brueggeman, R. and von Wettstein, D., May 2008). The sequence is contained in the BAC clone BAC 273i4, and encompasses 17 exons and 16 introns in a total size of 26642 bp. BAC 273i4 also contains 6300 bp 5′upstream from the ATG translational start and 6735 bp 3′downstream from the TAG translational stop codon. HvDME harbors 5946 bp of coding sequence which translates to a putative protein of 1981 aa. The DME-family amino acid sequences of Arabidopsis and other cereals were retrieved from the GenBank and Phytozome (Additional file 1: Table S1) and an alignment was constructed (Additional file 3). HvDME has a common structure with the other DME-type DNA glycosylases in that it harbours a lysine-rich region at the N-terminus, ensued by Domain A which is followed by the DNA glycosylase domain [including the helix-hairpin-helix motif (HhH) and the glycine-proline rich region flanked by a conserved aspartate (GPD)], the EndIII _4Fe-4Se domain and Domain B (Figure 1A and Additional file 3). HvDME has a high degree of homology with TaDME2 (AEF38424.1) (96.4% identity), BdDME (Bradi4g08870.1) (77.5% identity), OsDME (01 g11900.1) (70% identity), and less with ZmDME1 (GRMZM2G123587) (52% identity) and SbDME1 (08 g008620.1) (49% identity) (Additional file 3). Near absolute conservation is observed among cereals in the region spanning the DNA glycosylation domains and the the EndIII _4Fe-4Se domain. About 65% similarity exists between the DNA glycosylation domain of HvDME and the DNA glycosylation domain of AtDME (Additional file 3). The tertiary structure of the glycosylase domain of HvDME and AtDME, respectively, was predicted using Swissmodel (Figure 1B) and found to be very much alike except for a beta-strand loop in the HvDME, not predicted for the AtDME protein. The spatial orientation of the helix-hairpin-helix, the conserved aspartate (D), and the adjacent four-cysteine region binding a putative 4Fe-4S cluster were nearly identical (Figure 1B).
Schematic view of DME-family DNA glycosylases and predicted tertiary structure of HvDME and AtDME. A) Proteins, HvDME (FM164415), TaDME (AEF38424.1), BdDME (Bradi4g08870), AtDME (NP_196076.2), AtROS1 (AAP37178.1), AtDML2 (NP_187612.5) and AtDML3 (NP_195132.3) are depicted with white rectangles. White box, lysine-rich region; black box, glycosylase domain; hatched box, A domain; grey box, B domain; grey hatched box, 4Fe-4S binding domain; H-h-H, helix-hairpin-helix; GPD, glycine-proline rich region and conserved aspartate residue. B) Left: Amino acid sequence alignment of the glycosylase domain of DME-type proteins. Right: Predicted tertiary structure of HvDME and AtDME glycosylase domains. Helix-hairpin-helix is indicated with yellow arrows, the conserved aspartate (D) is shown in green and the 4Fe-4S is shown in orange-yellow.
A phylogenetic tree constructed using all known DME-family sequences from barley, wheat, brachypodium, rice, maize, sorghum and Arabidopsis showed that HvDME belongs in a cluster with the DME homologues from other cereals, all being more closely related to the AtDME, whereas is more distantly related to other DNA glycosylase members which group together with ROS1, DML2 and DML3 homologues (Figure 2). Barley homologues of the ROS1, DML2 and DML3 proteins have not been identified thus far.
Phylogenetic analysis of DME-family DNA glycosylases from monocots and dicots. Phylogenetic tree showing the classification of DME-type DNA glycosylases. The sequences used and their accession numbers are shown in Additional file 1: Table S1. Barley HvDME is in bold and Arabidopsis AtDME, AtROS1, AtDML2 and AtDML3 are grey-shaded. Numbers indicate bootstrap values (1000 = 100%).
Comparative genomic analysis of cereal DMEgenes
The HvDME gene sequence contained in the BAC clone BAC 273i4 has a total size of 26642 bp and harbors 17 exons and 16 introns (Figure 3A). HvDME also contains 6300 nt 5′upstream from the ATG translational start and 6735 nt 3′downstream from the TAG translational stop codon (Additional file 4 and Additional file 5). The gene sequences of Brachypodium BdDME (Bradi4g08870.1) and rice OsDME (01 g11900.1) also contain 17 exons and 16 introns, respectively. The exons are of similar sizes whereas some variation is observed among introns regarding relative size and position (Figure 3A and Additional file 4). ZmDME (GRMZM2G12358) consists of 16 exons and 15 introns with two large introns, 4, and 14. Using the MASiVE bioinformatics tool for detection of retroelements (http://tools.bat.ina.certh.gr/masive/) [51], two sites in the 3′downstream untranslated region of the HvDME gene were found to have a high degree of similarity with the PTAES_CS_cons_maximus Copia Sirevirus retroelement. Specifically, the 5′LTR region (1437 nt) and the 3′LTR region (1161 nt) of the PTAES_CS_cons_maximus element were detected at 1696 nt and 5540 nt downstream from the translational stop of HvDME, within 21600–23037 nt and 25444–26605 nt, respectively (Figure 3B and Additional file 5). Interestingly, a full length (9591 nt) maize Sirevirus retrotransposon, Copia Ji, was found to have high similarity with a fragment of intron 14 (from 34003 to 43593 nt) of the maize ZmDME1 (GRMZM2G123587) gene. Highest homology was with the 5′LTR and 3′LTR regions of the retrotransposon contained in 42135–43593 nt and 34003–35337 nt of the ZmDME1 gene (Figure 3A, 3B and Additional file 4). The Copia Ji retrotransposon was detected in four more sites of the maize genome as indicated in Figure 3B which upon further inspection in Ensemble (http://plants.ensembl.org/index.html) were found to be intergenic regions.
Genomic analysis of cereal DME genes. A) Schematic view of cereal HvDME (FM164415), BdDME (Bradi4g08870), OsDME (01 g11900.1) and ZmDME (GRMZM2G123587) genes. Exons are depicted with orange boxes and introns with blue lines. 3’ untranslated regions are shown as light blue lines. Regions within the 3’ untranslated region of HvDME and within the intron of ZmDME where retrotransposon sequences were found are highlighted in red. B) Sequence similarity of HvDME and ZmDME with Copia retrotransposons, visualized in Circoletto (http://tools.bat.ina.certh.gr/circoletto/) after blast analysis with MASiVE (http://tools.bat.ina.certh.gr/masive/) and LTRphyler (http://tools.bat.ina.certh.gr/ltrphyler/). Upper circle: Full length HvDME gene (left); the Copia PTAES_CS_cons_maximus Sirevirus retrotransposon (right). The 5’ and 3’ LTR regions of the retrotransposon are shown with bold black lines. The regions of retrotransposon homology between the LTRs and the 3’ downstream region of HvDME are marked in red. Lower circle: Full length ZmDME1 gene (right); the maize Copia Ji Sirevirus retrotransopson element in different chromosomal locations of the maize genome (left). The ZmDME gene resides at the Zmay_chr_5_D_1618643 site (chromosome 5, sense strand, position 1618643 bp from the chromosomal start). Regions of homology are shown in red. Coding regions of retrotransposon integrase and reverse transcriptase are in purple and yellow-green, respectively. Dark-grey and light- grey interconnecting ribbons depict regions of high and low similarity, respectively.
miRNA target analysis for DMEfrom barley and other cereals
The report that an Arabidopsis DME-family DNA glycosylase homologue, AtDML3, is regulated by the miRNA miR402 [52], prompted us to perform small RNA target analysis, in silico, for detecting putative small RNA targets on the HvDME gene sequences. Similar analysis was performed with the DME gene homologues from Brachypodium and rice using the psRNATarget (Plant small RNA Target) tool (http://plantgrn.noble.org/psRNATarget).
Inspection of the coding region of the DME genes did not identify target sites with sufficient complementarity to plant miRNAs. However, upon examining genomic sequences, near 100% complementarity was detected between miRNA HvmiR1126 and intron 15 (16644–19175 nt) of the HvDME gene sequence (Figure 4). 22 nt out of 23 nt of miRNA HvmiR1126 matched with the region 16838 nt-16860 nt of intron 15. Likewise near 100% complementarity was detected between the Brachypodium miRNA, BdmiR1122, and intron 15 of the BdDME gene and between the rice miRNA OsmiR1436 and intron 16 of the OsDME gene (Figure 4).
In silico miRNAs analysis. Putative miRNA target sites in HvDME (FM164415), BdDME (Bradi4g08870), and OsDME (01 g11900.1). MiRNA and target complementarities are depicted schematically above the sequences. White boxes represent exons; black lines represent introns and upstream and downstream regions. Identitical nucleotide matching in the miRNA-target hybrid is depicted by double dot (:). Permitted G-U matches are depicted by single dot (.).
Expression analysis of HvDMEin different tissues and seed developmental stages
Expression analysis of HvDME was examined in vegetative and reproductive tissues from the barley cultivar Kos (a medium seed-size cultivar) by qualitative RT-PCR (Figure 5). HvDME transcript is detectable in all tissues examined except from mature leaves and stamens. Highest expression of HvDME was in seedlings and in seeds 1–3 (DAF) which declined in seeds at 3–5 DAF.
Qualitative RT-PCR expression analysis of HvDME in vegetative and reproductive tissues in the cultivar Carina. 1, roots; 2, meristems; 3, 7 day-old seedlings; 4, leaves; 5, stamens; 6, immature flowers (before fertilization); 7, seeds 1–3 days after fertilization; 8, seeds 3–5 days after fertilization. HvActin was used as the positive control.
Expression analysis of HvDME in different seed developmental stages in two different cultivars with different seed size, Caresse (large-seed) and Ippolytos (small-seed), was performed by quantitative real time RT-PCR. In the cultivar Caresse, HvDME expression is higher by about 2 fold in the 5–10 DAF stage whereas it is lower by about 4 fold in the 10–15 DAF stage, as compared to the unfertilized immature flower (IF) (Figure 6). In the cultivar Ippolytos HvDME expression increased by about 2 fold in the 1–3 DAF seed stage as compared to the immature flower (IF) whereas in the later seed stages HvDME expression did not seem to change (Figure 6).
Quantitative real time RT-PCR analysis of HvDME during seed development in Caresse and Ippolytos. Expression values were normalized to those of HvActin. The relative expression ratio of each sample is compared to the control group which was immature flowers. IF, Immature flowers; 1–3, 3–5, 5–10, 10–15 DAF (seeds at 1–3, 3–5, 5–10, 10–15 days after fertilization). Data represent mean values from two independent experiments with standard deviations. Values significantly different (P < 0.05) from the control group (IF) are marked with an asterisk.
Expression analysis of HvDMEin response to drought stress
Environmental stress such as drought can have important consequences in proper plant development and seed yield. The drought response is a complex process involving the action of different structural genes and gene transcription factors [53, 54]. In addition epigenetic factors such as histone deacetylases, histone methyltransferases and demethylases, and miRNAs have been implicated in the response [44, 47, 55–58]. Therefore, another epigenetic regulator such as the DME gene may also be required for regulating the gene expression programmes participating in drought response. Expression of HvDME was investigated upon conditions of drought, in two barley cultivars with different tolerance to drought. Real-time PCR was employed to examine HvDME transcript accumulation in 7-d-old seedlings at 3 and 10 days after drought treatment in the drought-sensitive cultivar, Caresse, and the drought-tolerant cultivar, Demetra. A sound induction of ~ 10 fold was observed for HvDME, after 10 days of drought in the drought-tolerant cultivar Demetra as compared to the untreated control plants after 10 days (Figure 7). HvDME transcript levels increased by ~ 2 fold after 10 days of drought in the drought-sensitive cultivar Caresse (Figure 7).
Expression analysis of HvDME under drought. Quantitative real time PCR analysis of HvDME at 3 and 10 days after drought treatment of Caresse(drought-sensitive) and Demetra (drought-tolerant) 7-d-old seedlings. White bars, untreated plants; grey bars and light grey bars, drought-treated plants. Expression values were normalized to those of HvActin. Relative expression ratio of each sample was compared to the control group which was untreated plants, 3 days, and was assigned the value of 1. Data represent mean values from two independent experiments with standard deviations. Values significantly different (P < 0.05) from the untreated plants are marked with an asterisk.
DNA methylation analysis of the HvDMEgene
In order to examine the DNA methylation pattern of the HvDME gene and uncover potential links to gene expression differences between drought-treated and untreated plants, the DNA methylation profile of HvDME in control and drought conditions was analysed using an McrBC-PCR assay. McrBC is a methylation-sensitive restriction enzyme that cleaves DNA containing methylcytosine on one or both strands, recognizing two half-sites of the form (G/A)mC. After McrBC treatment, methylated DNA will be digested and will not be amplified by PCR, while unmethylated DNA will not be cleaved and will result in PCR product.
We analyzed Caresse and Demetra seedlings 10 days after drought treatment since this tissue showed the highest difference in expression (~2 fold and 10 fold increase, respectively) compared to untreated tissue (Figure 8). Four regions in the promoter of HvDME (regions 1, 2, 3 and 4), two coding regions (regions 6 and 7) and a region located 3780 nt upstream from the translation start site (region 5) were examined. No obvious bands regarding region 1 (−76 to −432 from the ATG start) and region 4 (−1381 to −1781) were detected, indicating that these regions are cytosine methylated in both cultivars. On the other hand, the presence of PCR bands for region 2 (−501 to −854) and 3 (−883 to −1167), both being part of a CpG island, suggests that they are unmethylated. Region 6 (+9 to +755 from the ATG start) containing the 5′end of exon 1 (747 nt), is methylated in both cultivars as indicated by the absence of PCR products. Interestingly, region 7 (containing part of exon 17 with 125 nt, and part of the 3′ untranslated region with 292 nt) is only methylated in Demetra, whereas a strong PCR band indicates that it is unmethylated in Caresse. Region 5 which is predicted to be a CpG island is heavily methylated in both Demetra and Caresse. Taken together, these data demonstrate the presence of cytosine methylation in both the promoter and gene-body regions of the HvDME gene in the two cultivars.
DNA methylation of HvDME. DNA methylation assays in 10 day drought-treated and untreated Caresse and Demetra seedlings. Upper: A schematic view of the HvDME gene depicted as a bold black line. Pale blue boxes, exons; pink boxes, LTRs. The regions used for McrBC-PCR are underlined and numbered. ATG and TAG codons are indicated. Lower: Analysis of PCR amplification of McrBC-digested and undigested genomic DNA on agarose gels. Genomic DNA was digested with McrBC and PCR amplification followed. (−), no McrBC; (+), digestion with McrBC.
Using the McrBc assay no differences in the DNA methylation status between control and drought-treated plants were detected in the regions examined in either cultivar suggesting that there may not be an association between DNA methylation and increased HvDME expression under drought conditions.
Two HvDME 3′ downstream regions (at 1696 nt and 5540 nt from the translational stop) found to contain the 5′ and 3′ LTR regions of a Copia retrotransposon, PTAES_CS_cons_maximus, were also examined for the presence of DNA methylation. The 5′ and 3′ LTRs were shown to be methylated in control and drought-treated plants in both cultivars (Figure 8).
Discussion
In the current study we present the sequence analysis, phylogenetic analysis, expression profiles, genomic organization, promoter analysis and DNA methylation patterns of a barley gene encoding a putative HvDME protein.
Sequence analysis
Comparison of amino acid sequences from different DME-family proteins from the dicot Arabidopsis and different monocot species revealed that the HvDME protein has a common modular structure as the other members of the DNA glycosylase superfamily. Furthermore, it contains the two unknown domains A and B, found only in DME-family DNA glycosylases. Phylogenetic analysis of DME-family sequences from different monocots revealed three major cereal clades representing homologues of the AtDME, AtROS1, and AtDML3 proteins. HvDME shows highest sequence similarity with the grass sequences, TaDME1 (AEF38423.1), TaDME2 (AEF38424.1) TaDME3 (AEF38425.1), BdDME (Bradi4g08870.1), and OsDME (01 g11900.1) which share over 70% identity and group together in a subcluster that is closer related to the Arabidopsis DME.
Analysis of the HvDME genomic organization, revealed a high degree of similarity with its grass homologues, BdDME (Bradi4g08870.1) and OsDME (01 g11900.1). The presence of sequences from an LTR Copia Sirevirus retroelement in the 3′ untranslated region of barley DME is consistent with the recent finding that 86% of the barley genome is composed of mobile elements or other repeat sequences the majority of which consists of LTR retrotransposons [59].
In Arabidopsis, the AtDML3 homologue has been suggested to be regulated by the stress-induced miRNA 402 during seed germination under stress conditions [52]. MiRNAs and small RNAs have been widely studied in plants in the past several years and have been shown to play important roles in various aspects of plant development [60]. Although initial studies focused on Arabidopsis, intense investigations were soon extended to agronomically important crops such as cereal monocots [57, 58, 61–64]. A search for miRNA targets on the HvDME sequence identified miRNA HvmiR1126 in intron 15 of the HvDME gene sequence at near 100% complementarily. Curiously, similar complementarity was detected between the Brachypodium miRNA, BdmiR1122, and intron 15 of the BdDME gene and between the rice miRNA, OsmiR1436, and the last intron of the OsDME gene, respectively. The high complementarity of these miRNAs and their targets might imply that they are functionally significant. Intronic miRNAs have been the object of recent studies and demonstrated to play important roles in the regulation of genes in mammals [65]. In plants, studies on intronic miRNAs have just started to emerge [66–68]. Certainly further investigations will be needed to unveil the possible significance of HvmiR1126.
Expressionanalysis
Our data on HvDME expression in barley vegetative, reproductive and seed tissues agrees with data retrieved from PLEXdb (http://www.plexdb.org/) concerning the expression of the DME gene from different barley cultivars such as Morex and Golden Promise showing DME mRNA presence throughout plant development. Moreover it concurrent with recent datasets from the large-scale genome and transcriptome analysis of barley [59] showing the HvDME transcript (MLOC_17707.1) present in Morex seedlings, developing tillers, immature flowers and 4 DAF embryos, and in seeds of 5 and 15 DAF. Ippolytos resembles Morex which is another small-seed cultivar (31.5 gr per thousand grains) as gene expression in 15 DAF seeds is about 1.5 higher than in 5 DAP seeds (ftp://ftpmips.helmholtz-muenchen.de/plants/barley/public_data/).
In barley, endosperm cellularization begins at approximately 4 DAF and ends at 6–8 DAF, when the seed maturation process begins [69, 70]. It might be possible that the differences in HvDME mRNA accumulation between Caresse (large-seed) and Ippolytos (small-seed) during these critical stages of endosperm development affect gene expression programmes associated with the processes of cellularization and seed filling and ultimately with the size of seed. At 10–15 DAF the barley seed has entered the maturation process with high starch synthesis rates and protein accumulation characterizing this seed storage stage. The differential expression of HvDME at the 10–15 DAF seed stage between the two cultivars may reflect varied requirements for metabolic enzyme-encoding genes playing roles in starch and protein synthesis and storage. Certainly the exact role of HvDME in the two cultivars will be elucidated by future functional analysis of the HvDME gene.
HvDME expression was examined under conditions of drought and was found to be substantially induced in drought-treated seedlings especially in the drought-tolerant cultivar. Interestingly, in the drought-tolerant cultivar, Demetra, there is a marked increase of HvDME expression of ~ 10 fold, after 10 days of drought, whereas only ~ 2 fold increase was observed for the drought-sensitive cultivar Caresse. This suggests a possible role for the HvDME gene in the drought response in a cultivar-dependent manner. Although studies on DME glycosylases under stress are currently scarce, a recent study of response to metal stress in rice revealed induction of the rice DME gene in soils with elevated mercury, cadmium and copper [71]. Perhaps induction of the DME gene is required in cereals in order to cope with the stress imposed on the plant by unfavourable soil composition and low water content.
DNA methylation
Three regions of the HvDME promoter (region 1, region 4, and region 5) were found to be methylated in both cultivars under control and drought conditions. On the other hand, two regions that are part of the CpG island (region 2 and region 3) of the HvDME promoter were unmethylated in all instances. It seems that the CpG island is in an unmethylated state while the neighbouring regions to the CpG island are methylated, which is in accordance with previous reports demonstrating that CpG islands are typically in a nonmethylated state in an otherwise heavily methylated genome [72]. The finding that an active gene is promoter-DNA methylated seems inconsistent with studies in Arabidopsis and rice that have associated promoter DNA methylation with gene repression [5, 73]. On the other hand, genome-wide mapping of DNA cytosine methylation in rice revealed that 8.1% of active genes were methylated within their promoter [74]. Similarly, the cold-induced gene ZmDREB1 in maize retains some cytosine methylation marks within the promoter region after cold induction [75]. In soybean, a region of the promoter of a salinity induced gene was demethylated upon salinity stress, whereas a neighbouring region remained hypermethylated supporting the suggestion that cytosine methylation is region specific [76]. In addition, it was reported that while cytosine methylation is a repressive mark, H3K4me2 alters the chromatin structure to a form permissive for initiation of transcription even in the presence of cytosine methylation [73]. In barley too, other factors such as histone modifications in interplay with promoter cytosine methylation may be critical in governing HvDME expression.
Gene-body (transcribed region) DNA methylation was also investigated for a fragment covering part of exon 1 and a fragment including exon 17 and a part of the 3′UTR. Exon 1 was shown to be methylated in both Demetra and Caresse, whereas exon 17 was methylated only in Demetra. DNA methylation in HvDME gene-body is consistent with a number of reports demonstrating the presence of gene-body methylation in plants. DNA methylation within transcribed regions has been detected in about a third of Arabidopsis [77, 78] and rice [73, 79] genes. Substantial expression of HvDME in various tissues and its enrichment for gene-body DNA methylation is in accordance to previous studies in Arabidopsis showing that highly and moderately expressed genes are more likely to be DNA methylated within the gene-body region [77, 80]. Additionally, it is in agreement with a recent study where the single-base methylome of wild- and cultivated- rice revealed that promoter DNA methylation is associated with gene repression whereas gene-body DNA methylation is associated with gene expression [79].
Apart from its importance in seed development and stress copying mechanisms, understanding the regulation of DME genes could have important implications in nutrition and health. In a very recent investigation attempting to analyze the effects of wheat and barley DME genes on the levels of immunoactive prolamins associated with gluten-intolerance and celiac disease, wheat DME was RNAi-downregulated resulting in decreased expression of endosperm prolamins [39]. These efforts could lead to improved cereal cultivars producing safe cereal products for gluten-sensitive individuals [34–39].
During the past several years our group has studied different epigenetic chromatin regulators implicated in gene activation or gene repression in barley [40–48]. Ongoing efforts to further our knowledge on epigenetic regulatory mechanisms impacting seed development, nutritional seed content, and the plants′ resistance to abiotic stresses such as drought, could lead to breeding for improved Triticeae varieties.
Conclusions
A DME homologue was characterized in barley and the encoded protein was found to group together with other cereal DME-family proteins more closely related to AtDME and more distantly related to AtROS1, AtDML2 and AtDML3. HvDME contains remnants of a Copia retroelement in its 3′downstream region which maybe important for its regulation. HvDME displayed differential expression during seed development in two cultivars varying in seed size, implying a role in endosperm development and seed maturation. Moreover, HvDME expression is markedly increased in dehydrated seedlings in a drought-tolerant cultivar pointing to a role in response to abiotic stress such as drought. Finally, differential DNA methylation in different regions of the gene-body in two different cultivars suggests epigenetic regulation of the HvDME gene. The study of a barley DME gene will contribute to our understanding of epigenetic regulation during seed development and in response to abiotic stresses in cereal crops of high agronomic value.
References
Bennetzen JL, Zhu JK: Epigenetics of the epigenome. Curr Opin Plant Biol. 2011, 14 (2): 3-3.
De Lucia F, Dean C: Long non-coding RNAs and chromatin regulation. Curr Opin Plant Biol. 2011, 14 (2): 168-173. 10.1016/j.pbi.2010.11.006.
Moazed D: Mechanisms for the inheritance of chromatin states. Cell. 2011, 146 (4): 510-518. 10.1016/j.cell.2011.07.013.
Feng S, Cokus S, Zhang X, Chen P, Bostick M, Goll M, Hetzel J, Jain J, Strauss S, Halpern M: Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci. 2010, 107: 8689-8694. 10.1073/pnas.1002720107.
Law JA, Jacobsen SE: Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010, 11 (3): 204-220. 10.1038/nrg2719.
Zhu J-K: Active DNA demethylation mediated by DNA glycosylases. Annu Rev Genet. 2009, 43: 66-143.
Gehring M, Reik W, Henikoff S: DNA demethylation by DNA repair. Trends Genet. 2009, 25: 82-90. 10.1016/j.tig.2008.12.001.
Wu SC, Zhang Y: Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010, 11: 607-620. 10.1038/nrm2950.
Guo Junjie U, Su Y, Zhong C, Ming G-l, Song H: Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011, 145 (3): 423-434. 10.1016/j.cell.2011.03.022.
He Y-F, Li B-Z, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al: Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011, 333 (6047): 1303-1307. 10.1126/science.1210944.
Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PAC, Rappsilber J, Helin K: TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011, 473 (7347): 343-348. 10.1038/nature10066.
Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL: DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell. 2002, 110 (1): 33-42. 10.1016/S0092-8674(02)00807-3.
Gehring M, Huh JH, Hsieh T-F, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL: DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006, 124 (3): 495-506. 10.1016/j.cell.2005.12.034.
Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-MarΓn MI, MartΓnez-MacΓas MI, Ariza RR, RoldΓ΅n-Arjona T: DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci USA. 2006, 103 (18): 6853-6858. 10.1073/pnas.0601109103.
Ponferrada-Marin MI, Roldan-Arjona T, Ariza RR: ROS1 5-methylcytosine DNAglycosylase is a slow-turnover catalyst that initiates DNA demethylation in a distributive fashion. Nucleic Acids Res. 2009, 37: 4264-4274. 10.1093/nar/gkp390.
Bruner SD, Norman DPG, Verdine GL: Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000, 403: 859-866. 10.1038/35002510.
Guan Y, et al: MutY catalytic core, mutant and bound adenine structures define specificity for DNA repair enzyme superfamily. Nat Struct Biol. 1998, 5: 1058-1064. 10.1038/4168.
Mok YG, Uzawa R, Lee J, Weiner GM, Eichman BF, Fischer RL, Huh JH: Domain structure of the DEMETER 5-methylcytosine DNA glycosylase. Proc Natl Acad Sci. 2010, 107 (45): 19225-19230. 10.1073/pnas.1014348107.
Agius F, Kapoor A, Zhu J-K: Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc Natl Acad Sci. 2006, 103 (31): 11796-11801. 10.1073/pnas.0603563103.
Penterman J, Uzawa R, Fischer RL: Genetic interactions between DNA demethylation and methylation in Arabidopsis. Proc Natl Acad Sci USA. 2007, 145 (4): 1549-1557.
Penterman J, Zilberman D, Huh J, Ballinger T, Henikoff S, Fischer R: DNA demethylation in the Arabidopsis genome. Proc Natl Acad Sci USA. 2007, 104: 6752-6757. 10.1073/pnas.0701861104.
Jiang H, Kohler C: Evolution, function, and regulation of genomic imprinting in plant seed development. J Exp Bot. 2012, 63 (13): 4713-4722. 10.1093/jxb/ers145.
Kohler C, Wolff P, Spillane C: Epigenetic mechanisms underlying genomic imprinting in plants. Annu Rev Plant Biol. 2012, 63 (1): 331-352. 10.1146/annurev-arplant-042811-105514.
Bauer MJ, Fischer RL: Genome demethylation and imprinting in the endosperm. Curr Opin Plant Biol. 2011, 14 (2): 162-167. 10.1016/j.pbi.2011.02.006.
Kohler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U: The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 2003, 17: 1540-1553. 10.1101/gad.257403.
Makarevich G, Villar CBR, Erilova A, Kohler C: Mechanism of PHERES1 imprinting in Arabidopsis. J Cell Sci. 2008, 121: 906-912. 10.1242/jcs.023077.
Gehring M, Bubb K, Henikoff S: Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009, 324: 1447-1451. 10.1126/science.1171609.
Hsieh T-F, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D: Genome-wide demethylation of Arabidopsis endosperm. Science. 2009, 324 (5933): 1451-1454. 10.1126/science.1172417.
Ibarra CA, Feng X, Schoft VK, Hsieh T-F, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, et al: Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science. 2012, 337 (6100): 1360-1364. 10.1126/science.1224839.
Calarco Joseph P, Borges F, Donoghue Mark TA, Van Ex F, Jullien Pauline E, Lopes T, Gardner R, Berger F, Feijo Jose A, Becker Jorg D, et al: Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012, 151 (1): 194-205. 10.1016/j.cell.2012.09.001.
Schoft VK, Chumak N, Choi Y, Hannon M, Garcia-Aguilar M, Machlicova A, Slusarz L, Mosiolek M, Park J-S, Park GT, et al: Function of the DEMETER DNA glycosylase in the Arabidopsis thaliana male gametophyte. Proc Natl Acad Sci. 2011, 108 (19): 8042-8047. 10.1073/pnas.1105117108.
La H, Ding B, Mishra GP, Zhou B, Yang H, Bellizzi MR, Chen S, Meyers BC, Peng Z, Zhu J-K, et al: A 5-methylcytosine DNA glycosylase/lyase demethylates the retrotransposon Tos17 and promotes its transposition in rice. Proc Natl Acad Sci. 2011, 108 (37): 15498-15503. 10.1073/pnas.1112704108.
Ono A, Yamaguchi K, Fukada-Tanaka S, Terada R, Mitsui T, Iida S: A null mutation of ROS1a for DNA demethylation in rice is not transmittable to progeny. Plant J. 2012, 4: 564-574.
Von Wettstein D: Mutants pave the way to wheat and barley for celiac patients and dietary health. 2009, Induced plant mutations in the genomics era. Food and Agriculture organization of the United Nations, Rome: Edited by Shu QY, 187-190. [ISBN: 978-92-5-106324-8]
von Wettstein D, Rustgi S, Kannangara C, Ankrah N, Wen S, Brew-Appiah R, Wen N, Gemini R, Brueggeman R, Reisenauer P, et al: A multipronged approach to develop nutritionally improved, celiac safe, wheat cultivars. Annu Wheat Newslet. 2010, 56: 261-264.
Langen G, Kogel K, Von Wettstein D: Mirror of Research University of Giessen, No 1, May 2011. Gluten Free Wheat - New Hope Forceliac Patients. 2011, http://geb.uni-giessen.de/geb/volltexte/2011/8116/pdf/SdF_2011_01_12_19.pdf,
Osorio C, Wen N, Gemini R, Zemetra R, von Wettstein D, Rustgi S: Targeted modification of wheat grain protein to reduce the content of celiac causing epitopes. Funct Integr Genomics. 2012, 12: 417-438. 10.1007/s10142-012-0287-y. PMID: 22732824
Rustgi S, von Wettstein D, Ankrah N, Brew-Appiah R, Wen S, Wen N, Osorio C, Gemini R, Reisenauer P, Lu X, et al: Engineering wheat for celiac patients. Ann Wheat Newslet. 2012, 58: 248-253.
Wen S, Wen N, Pang J, Langen G, Brew-Appiah RAT, Mejias JH, Osorio C, Yang M, Gemini R, Moehs CP, et al: Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc Natl Acad Sci. 2012, 109 (50): 20543-20548. 10.1073/pnas.1217927109.
Demetriou K, Kapazoglou A, Bladenopoulos K, Tsaftaris A: Epigenetic chromatin modifiers in barley: II. Characterization and expression analysis of the HDA1 family of barley histone deacetylases during development and in response to jasmonic acid. Plant Mol Biol Report. 2010, 28 (1): 9-21. 10.1007/s11105-009-0121-4.
Demetriou K, Kapazolgou A, Tondelli A, Francia E, Stanca MS, Bladenopoulos K, Tsaftaris AS: Epigenetic chromatin modifiers in barley: I. Cloning, mapping and expression analysis of the plant specific HD2 family of histone deacetylases from barley, during seed development and after hormonal treatment. Physiol Plant. 2009, 136: 358-368. 10.1111/j.1399-3054.2009.01236.x.
Kapazoglou A, Engineer C, Drosou V, Kalloniati C, Tani E, Tsaballa A, Kouri E, Ganopoulos I, Flemetakis E, Tsaftaris A: The study of two barley type I-like MADS-box genes as potential targets of epigenetic regulation during seed development. BMC Plant Biol. 2012, 12 (1): 166-10.1186/1471-2229-12-166.
Kapazoglou A, Tondelli A, Papaefthimiou D, Ampatzidou H, Francia E, Stanca M, Bladenopoulos K, Tsaftaris A: Epigenetic chromatin modifiers in barley: IV. The study of barley polycomb group (PcG) genes during seed development and in response to external ABA. BMC Plant Biol. 2010, 10 (1): 73-10.1186/1471-2229-10-73.
Papaefthimiou D, Tsaftaris AS: Characterization of a drought inducible trithorax-like H3K4 methyltransferase from barley. Biol Plant. 2012, 56 (4): 683-692. 10.1007/s10535-012-0125-z.
Papaefthimiou D, Kapazoglou A, Tsaftaris A: Cloning and characterization of SOC1 homologues in barley (hordeum vulgare) and their expression during seed development and in response to vernalization. Physiol Plant. 2012, doi:10.1111/j.1399-3054.2012.01610.x
Papaefthimiou D, Likotrafiti E, Kapazoglou A, Bladenopoulos K, Tsaftaris A: Epigenetic chromatin modifiers in barley: III, isolation and characterization of the barley GNAT-MYST family of histone acetyltransferases and responses to exogenous ABA. Plant Physiol Biochem. 2010, 48 (2–3): 98-107.
Papaefthimiou D, Tsaftaris A: Significant induction by drought of HvPKDM7-1, a gene encoding a jumonji-like histone demethylase homologue in barley (H. Vulgare). Acta Physiol Plant. 2012, 34: 1187-1198. 10.1007/s11738-011-0915-5.
Tsaftaris AS, Kapazoglou A, Darzentas N: Epigenetics, epigenomics, and implications in plant breeding. “Plant Biotechnology and Agriculture: Prospects for the 21st Century”. Edited by: Altman A, Haegawa PM. Elsevier Press: Altman A, Haegawa PM; 2012:207-226.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011, 28 (10): 2731-2739. 10.1093/molbev/msr121.
Saitou N, Nei M: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987, 4: 406-
Darzentas N, Bousios A, Apostolidou V, Tsaftaris AS: MASiVE: mapping and analysis of SireVirus elements in plant genome sequences. Bioinformatics. 2010, 26 (19): 2452-2454. 10.1093/bioinformatics/btq454.
Kim JY, Kwak KJ, Jung HJ, Lee HJ, Kang H: MicroRNA402 Affects seed germination of Arabidopsis thaliana under stress conditions via targeting DEMETER-LIKE Protein3 mRNA. Plant Cell Physiol. 2010, 51 (6): 1079-1083. 10.1093/pcp/pcq072.
Guo P, Baum M, Grando S, Ceccarelli S, Bai G, Li R, von Korff M, Varshney RK, Graner A, Valkoun J: Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J Exp Bot. 2009, 60 (12): 3531-3544. 10.1093/jxb/erp194.
Hirayama T, Shinozaki K: Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010, 61 (6): 1041-1052. 10.1111/j.1365-313X.2010.04124.x.
Ding Y, Tao Y, Zhu C: Emerging roles of microRNAs in the mediation of drought stress response in plants. J Exp Bot. 2013, 64 (11): 3077-3086. 10.1093/jxb/ert164.
Hu Y, Qin F, Huang L, Sun Q, Li C, Zhao Y, Zhou D-X: Rice histone deacetylase genes display specific expression patterns and developmental functions. Biochem Biophys Res Commun. 2009, 388 (2): 266-271. 10.1016/j.bbrc.2009.07.162.
Kantar M, Lucas S, Budak H: miRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta. 2011, 233 (3): 471-484. 10.1007/s00425-010-1309-4.
Kantar M, Unver T, Budak H: Regulation of barley miRNAs upon dehydration stress correlated with target gene expression. Funct Integr Genomics. 2010, 10 (4): 493-507. 10.1007/s10142-010-0181-4.
The International Barley Genome Sequencing Consortium: A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012, 491 (7426): 711-716.
Voinnet O: Origin, biogenesis, and activity of plant MicroRNAs. Cell. 2009, 136 (4): 669-687. 10.1016/j.cell.2009.01.046.
Curaba J, Spriggs A, Taylor J, Li Z, Helliwell C: miRNA regulation in the early development of barley seed. BMC Plant Biol. 2012, 12 (1): 120-10.1186/1471-2229-12-120.
Schreiber A, Shi B-J, Huang C-Y, Langridge P, Baumann U: Discovery of barley miRNAs through deep sequencing of short reads. BMC Genomics. 2011, 12 (1): 129-10.1186/1471-2164-12-129.
Zhang L, Chia J-M, Kumari S, Stein JC, Liu Z, Narechania A, Maher CA, Guill K, McMullen MD, Ware D: A genome-wide characterization of MicroRNA genes in maize. PLoS Genet. 2009, 5 (11): e1000716-10.1371/journal.pgen.1000716.
Li D, Wang L, Liu X, Cui D, Chen T, Zhang H, Jiang C, Xu C, Li P, Li S, et al: Deep sequencing of maize small RNAs reveals a diverse Set of MicroRNA in Dry and imbibed seeds. PLoS ONE. 2013, 8 (1): e55107-10.1371/journal.pone.0055107.
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM: MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010, 328 (5985): 1566-1569. 10.1126/science.1189123.
Yan K, Liu P, Wu C-A, Yang G-D, Xu R, Guo Q-H, Huang J-G, Zheng C-C: Stress-induced alternative splicing provides a mechanism for the regulation of MicroRNA processing in Arabidopsis thaliana. Mol Cell. 2012, 48 (4): 521-531. 10.1016/j.molcel.2012.08.032.
Yang GD, Yan K, Wu BJ, Wang YH, Gao YX, Zheng CC: Genome wide analysis of intronic microRNAs in rice and Arabidopsis. J Genet. 2012, 91 (3): 313-324. 10.1007/s12041-012-0199-6.
Meng Y, Shao C: Large-scale identification of mirtrons in Arabidopsis and rice. PLoS ONE. 2012, 7 (2): e31163-10.1371/journal.pone.0031163.
Agarwal P, Kapoor S, Tyagi AK: Transcription factors regulating the progression of monocot and dicot seed development. Bioessays. 2011, 33 (3): 189-202. 10.1002/bies.201000107.
Sabelli PA, Larkins BA: The development of endosperm in grasses. Plant Physiol. 2009, 149 (1): 14-26. 10.1104/pp.108.129437.
Ou X, Zhang Y, Xu C, Lin X, Zang Q, Zhuang T, Jiang L, Von Wettstein D, Liu B: Transgenerational inheritance of modified DNA methylation patterns and enhanced tolerance induced by heavy metal stress in rice (oryza sativa L.). PLoS ONE. 2012, 7 (9): e41143-10.1371/journal.pone.0041143.
Deaton AM, Bird A: CpG islands and the regulation of transcription. Genes Dev. 2011, 25 (10): 1010-1022. 10.1101/gad.2037511.
Li X, Wang X, He K, Ma Y, Su N, He H, Stolc V, Tongprasit W, Jin W, Jiang J: High-resolution mapping of epigenetic modifications of the rice genome uncovers interplay between DNA methylation, histone methylation, and gene expression. Plant Cell. 2008, 20: 259-276. 10.1105/tpc.107.056879.
Yan H, Kikuchi S, Neumann P, Zhang W, Wu Y, Chen F, Jiang J: Genome-wide mapping of cytosine methylation revealed dynamic DNA methylation patterns associated with genes and centromeres in rice. Plant J. 2010, 63 (3): 353-365. 10.1111/j.1365-313X.2010.04246.x.
Hu Y, Zhang LU, He S, Huang MIN, Tan J, Zhao LIN, Yan S, Li HUI, Zhou KUN, Liang Y, et al: Cold stress selectively unsilences tandem repeats in heterochromatin associated with accumulation of H3K9ac. Plant Cell Environ. 2012, 35 (12): 2130-2142. 10.1111/j.1365-3040.2012.02541.x.
Song Y, Ji D, Li S, Wang P, Li Q, Xiang F: The dynamic changes of DNA methylation and histone modifications of salt responsive transcription factor genes in soybean. PLoS ONE. 2012, 7 (7): e41274-10.1371/journal.pone.0041274.
Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SWL, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, et al: Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006, 126 (6): 1189-1201. 10.1016/j.cell.2006.08.003.
Cokus S, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild C, Pradhan S, Nelson S, Pellegrini M, Jacobsen S: Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008, 452: 215-219. 10.1038/nature06745.
Li X, Zhu J, Hu F, Ge S, Ye M, Xiang H, Zhang G, Zheng X, Zhang H, Zhang S, et al: Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genomics. 2012, 13 (1): 300-10.1186/1471-2164-13-300.
Zilberman D, Gehring M, Tran R, Ballinger T, Henikoff S: Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007, 39: 61-69. 10.1038/ng1929.
Acknowledgments
We would like to thank Dr Konstantinos Bladenopoulos (NAGREF) for providing seed material. This work was supported by a PENED grant (Ο3Ε_402/2003), the EU-COST ACTION 406 and the project AMYLO (SYN-22-878). Continuous support for the Institute of Applied Biosciences/CERTH from the General Secretariat of Research and Technology of Greece is also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AK conceived and designed the experiments, performed RNA isolation, qualitative and quantitative real time PCR assays, protein sequence analysis, phylogenetic analysis, genomic organization analysis and wrote the manuscript. VD performed promoter analysis, designed and performed DNA methylation experiments and participated in the writing of the manuscript. AA participitated in the DNA methylation studies. AST revised the manuscript and directed the whole study. All authors read and approved the final manuscript.
Electronic supplementary material
12870_2013_1780_MOESM3_ESM.doc
Additional file 3: Amino acid sequence alignment of DME protein sequences from Hordeum vulgare , HvDME (FM164415.1); Triticum aestivum , Ta DME2 (AEF38424.1); Brachypodium distachyon , BdDME (Bradi4g08870.1); Oryza sativa , OsDME (01 g11900.1); Zea Mays , ZmDME (GRMZM2G123587); Sorghum bicolor , SbDME (08 g008620.1); Arabidopsis thaliana , AtDME, AtROS1, AtDML2, AtDML3. Identical amino acids are shown with asterisks and similar amino acids with dots. The DNA glycosylase domain is indicated in grey highlights, the helix-hairpin-helix region is overlined and marked in purple, the GPD region is overlined and the conserved aspartic acid residue is marked in green. The four cysteine residues forming the 4Fe-4S cluster are shown in red. The conserved lysine-rich region, and the A and B regions are also overlined. (DOC 107 KB)
12870_2013_1780_MOESM5_ESM.doc
Additional file 5: The sequence of clone BAC 273i4, containing the HvDME gene. ATG and TAG translational start and stop codons, respectively, are shaded in pink. 5’ and 3’ LTR regions of the Copia sirevirus retrotransposon PTAES_CS_cons_maximus are shaded in grey. (DOC 74 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kapazoglou, A., Drosou, V., Argiriou, A. et al. The study of a barley epigenetic regulator, HvDME,in seed development and under drought. BMC Plant Biol 13, 172 (2013). https://doi.org/10.1186/1471-2229-13-172
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2229-13-172