Abstract
Background
The diphtheria toxin repressor, DtxR, of Corynebacterium diphtheriae has been shown to be an iron-activated transcription regulator that controls not only the expression of diphtheria toxin but also of iron uptake genes. This study aims to identify putative binding sites and operons controlled by DtxR to understand the role of DtxR in patho-physiology of Corynebacterium diphtheriae.
Result
Positional Shannon relative entropy method was used to build the DtxR-binding site recognition profile and the later was used to identify putative regulatory sites of DtxR within C. diphtheriae genome. In addition, DtxR-regulated operons were also identified taking into account the predicted DtxR regulatory sites and genome annotation. Few of the predicted motifs were experimentally validated by electrophoretic mobility shift assay. The analysis identifies motifs upstream to the novel iron-regulated genes that code for Formamidopyrimidine-DNA glycosylase (FpG), an enzyme involved in DNA-repair and starvation inducible DNA-binding protein (Dps) which is involved in iron storage and oxidative stress defense. In addition, we have found the DtxR motifs upstream to the genes that code for sortase which catalyzes anchoring of host-interacting proteins to the cell wall of pathogenic bacteria and the proteins of secretory system which could be involved in translocation of various iron-regulated virulence factors including diphtheria toxin.
Conclusions
We have used an in silico approach to identify the putative binding sites and genes controlled by DtxR in Corynebacterium diphtheriae. Our analysis shows that DtxR could provide a molecular link between Fe+2-induced Fenton's reaction and protection of DNA from oxidative damage. DtxR-regulated Dps prevents lethal combination of Fe+2 and H2O2 and also protects DNA by nonspecific DNA-binding. In addition DtxR could play an important role in host interaction and virulence by regulating the levels of sortase, a potential vaccine candidate and proteins of secretory system.
Similar content being viewed by others
Background
Iron is an important inorganic component of a cell. Iron is required as co-factor for various essential enzymes and proteins some of which are involved in electron transport (Cytochromes), redox reactions (oxidoreductases) and regulation of gene expression (fumarate-nitrate reduction regulatory protein, iron-binding protein) [1]. However a higher level of intracellular iron can catalyze formation of hydroxyl radicals and reactive oxygen species through Fenton's reaction which could be lethal to the cell [2]. Hence, a careful regulation of iron-requiring enzymes/proteins and iron uptake proteins/enzymes is required for the survival of bacteria.
Inorganic iron is also known to influence virulence in many pathogenic bacteria such as Corynebacterium diphtheriae, Escherichia coli, and Bordetella bronchiseptica [3–5]. The diphtheria toxin repressor DtxR is known as an iron-activated global transcription regulator that represses the transcription of various iron-dependent genes in C. diphtheriae [6, 7]. Eight DtxR-binding sites in upstream sequences of operons/genes named as tox, hmuO, irp1, irp2, irp3, irp4, irp5 and irp6 have been identified by DNA footprinting methods [6]. The product of tox gene is diphtheria toxin which catalyzes the NAD-dependent ADP ribosylation of eukaryotic aminoacyl-transferase-II, thereby causing inhibition of protein synthesis and subsequent death of the host. The hmuO gene, which encodes a haem oxygenase, oxidizes the haem to release free iron. The operons irp1 and irp6 encode the products with homology to ABC-type ferric-siderophore transport systems. The gene irp3 encodes a homologue of AraC-type transcriptional activators. The products of irp2, irp4 and irp5 do not show any homology to the other known proteins. In addition, C. diphtheriae with inactive DtxR has been shown to be sensitive to killing by exposure to high iron conditions or hydrogen peroxide than the wild type [8].
This work uses an in silico method to identify additional DtxR-binding sites and target genes to understand the role of DtxR in virulence and patho-physiology of C. diphtheriae.
Results
In silico identification of putative DtxR-binding sites
Experimentally characterized DtxR-binding motifs were collected from the literature (Table 1). These binding sites were used to identify additional putative DtxR-binding sites along with associated operons in C. diphtheriae NCTC13129 genome (see materials and methods). Table 2 shows the predicted DtxR-binding sites with score 3.7438 or more. We could identify five (tox, irp4, irp5, irp6 and hmuO) of the eight known DtxR-binding sites, in sequenced C. diphtheriae NCTC13129 genome. We could not find irp1 and irp2 motifs as the corresponding genes (irp1, irp2) are not present in the sequenced strain NCTC13129 [9]. The regulator binding sites of irp3, irp4 and irp6 genes in the strain NCTC13129 shows one base change from the binding sites reported in strain C7 [6]. Binding site of irp3 gene (TTAGGTGAGACGCACCCAT) although exists in strain NCTC13129, but not there in the predicted sites, because it is located within the coding region of irp3 ORF. The predicted ORF of irp3 in the sequenced strain NCTC13129 has different start position and is larger than what was previously reported in strain C7 [9, 10].
In addition, we have identified binding sites in upstream sequences of eight genes recently reported to be regulated by DtxR [7]. However, our prediction differs from the previous report for five (secY, deoR, chtA, frgA, sidA) of the seven sites which were identified by BLAST search (Table 2). Our prediction agreed with the previous report that the genes such as recA (DIP1450) and ywjA (DIP1735) are not under a direct DtxR regulation as we could not detect any motif upstream to these gene with scores above the cutoff value [7].
Experimental validation of predicted binding sites
Since our approach to identify DtxR-regulated genes is purely computational in nature, we decided to test the validity of our predictions. A sample of predicted regulator binding motifs (Table 2) (upstream to ORFs: DIP2161, DIP0699, DIP0586, DIP2304, DIP2272) were experimentally verified by EMSA using IdeR, an orthologue of DtxR from M. tuberculosis. DtxR and IdeR are iron-dependent regulators. A pair wise sequence comparison of the two proteins shows a high (58%) overall sequence identity (similarity 72%) which increases further to 92% identity and 100% similarity in DNA recognition domain. In addition, the structural comparison of two regulators also shows a very similar 3D organization, suggesting that the IdeR regulator would be able to recognize the DtxR motif [11].
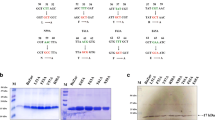
Synthetic double stranded oligonucleotides corresponding to DNA-binding sites were labeled with 32P and mixed with purified IdeR in presence of manganese ions and was assayed for the formation of DNA-protein complex using EMSA. Manganese was used as the divalent metal in the binding reactions on account of its redox stability compared with ferrous ion. Electrophoretic mobility of all five double stranded oligonucleotides tested was retarded by IdeR (Figure 1). However a synthetic motif (TTTTCATGACGTCTTCTAA) used as a negative control did not show any complex formation. These results indicate that the predicted DtxR-binding sites can indeed bind to DtxR.
IdeR binds the predicted DtxR-binding DNA fragments. 30 pmoles of IdeR was added to 32P-labelled DNA probes in the presence of 200 μM Mn2+, and complexes were resolved on a 7% Tris-borate polyacrylamide gel containing 150 μM Mn2+. Lane 1: Control gel retardation using Radiolabeled DNA motif without DtxR-binding site. Lane 2: Radiolabeled DIP2161 motif without IdeR. Lane 3: Radiolabeled DIP2161 motif with IdeR. Lane 4: Radiolabeled DIP0699 motif with IdeR. Lane 5: Radiolabeled DIP0586 motif with IdeR. Lane 6: Radiolabeled DIP2304 motif with IdeR. Lane 7: Radiolabeled DIP2272 motif with IdeR.
Identification and annotation of DtxR-regulated genes C. diphtheriae genome
In addition to the binding site prediction, we have also identified co-regulated genes (operons) downstream to the predicted DtxR-binding site (Table 3). Function of the proteins encoded by the putative genes in Table 2 and Table 3 was predicted by RPS-BLAST search against conserved domain database [12].
Discussion
Our analysis identified putative DtxR motifs upstream to various operons/genes which could be involved in siderophore biosynthesis, ABC-type transport systems, iron storage, oxidative stress defense and iron-sulfur cluster biosynthesis. In addition, we have also identified the motifs upstream of operons that could be involved in anchoring of host-interacting proteins to the cell wall and secretion of various virulence factors. Important functions of some of these DtxR-regulated genes and their role in C. diphtheriae physiology are discussed here.
Regulation of siderophore biosynthesis and ABC-type transport systems
Predicted member of the DtxR regulon, the gene DIP0586, codes for the IucA/IucC family of enzymes that catalyze discrete step in the biosynthesis of the aerobactin [13]. In addition to known DtxR-regulated siderophore transport genes (irp1, irp6), DtxR could also regulate other ABC-type transport systems similar to Manganese/Zinc, peptide/Nickel and multidrug subfamilies of ABC transporters. The peptide/nickel transport system (DIP2162-DIP2165) has been suggested to be recently acquired by pathogenic C. diphtheriae [9].
Regulation of iron storage and oxidative stress defense
We predict that DtxR could regulate divergently transcribed genes DIP2303 and DIP2304 whose products are similar to starvation inducible DNA-binding protein (Dps) and Formamidopyrimidine-DNA glycosylase (Fpg), respectively. Dps in Escherichia coli is induced in response to oxidative or nutritional stress and protects DNA from oxidative stress damage by nonspecific binding [14]. Dps also catalyzes oxidation of ferrous iron to ferric iron by hydrogen peroxide (2Fe2+ + H2O2 + 2H2O → 2Fe+3OOH(core) + 4H+) which in turn prevents hydroxyl radical formation by Fenton's reaction (Fe2+ + H2O2 → Fe+3 + HO- + HO.) and thereby prevents subsequent DNA damage [15]. The enzyme, formamidopyrimidine-DNA glycosylase is a primary participant in the repair of 8-oxoguanine, an abundant oxidative DNA lesion [16]. The gene DIP1510 which codes for the site-specific recombinase XerD could also be regulated by DtxR. The xerD gene in E. coli belongs to the oxidative stress regulon [17].
Regulation of proteins involved in iron-sulfur cluster biosynthesis and iron-sulfur cluster containing proteins
We predict that the operon DIP1288-DIP1296, which is similar to the suf operon of E. coli, could be regulated by DtxR. The suf operon in bacteria encodes the genes for Fe-S cluster assembly machinery [18]. In addition, genes encoding the iron-sulfur containing proteins such as succinate dehydrogenase (Sdh), cytochrome oxidase (CtaD) and Ribonucleotide reductase (NrdF1) in C. diphtheriae also show DtxR motif in their upstream sequences.
Regulation of sortases
We predict that DtxR could regulate the recently acquired pathogenic island DIP2271-DIP2272, encoding the sortase srtA and hypothetical protein, respectively [9]. Sortases are membrane-bound trans-peptidases that catalyze the anchoring of surface proteins to the cell wall peptidoglycan [9]. Such systems are often used by gram-positive pathogens to anchor host-interacting proteins to the bacterial surface [19].
Regulation of protein translation and translocation system
DtxR could regulate two operons that contain genes DIP0699 (secA) and DIP0540 (secY) that code for the protein translocation system. The sec Y-containing operon, which is similar to the streptomycine operon spc from B. subtilis and other bacteria, involves the genes required for protein translation and translocation [20]. The operon contains additional sialidase gene (DIP0543) in comparison to non pathogenic Corynebacterium species. Activity of sialidase has been linked to virulence in several other microbial pathogens and may enhance fimbriae mediated adhesion in Corynebacterium diphtheriae by unmasking receptors on mammalian cells [9].
The Sec system can both translocate proteins across the cytoplasmic membrane and insert integral membrane proteins into it. The former proteins but not the latter possess N-terminal, cleavable, targeting signal sequences that are required to direct the proteins to the Sec system. Some of the DtxR-regulated genes including diphtheria toxin (Table 4) show predicted signal sequences by SignalP 3.0 [21] and hence they may play an important role in host interaction and virulence of Corynebacterium diphtheriae [9].
Conclusions
The bioinformatics method used to predict the targets of DtxR in C. diphtheriae NCTC13129 genome is promising, as some of the predicted targets were experimentally verified. The approach identified novel DtxR-regulated genes, which could play an important role in physiology of C. diphtheriae NCTC13129. DtxR, generally known as a repressor of diphtheriae toxin and iron siderophore/transport genes, can also regulate other metal ion transport genes, iron storage, oxidative stress, DNA-repair, biosynthesis of iron-sulfur cluster, Fe-S-cluster containing proteins, and even protein sortase and translocation systems.
Methods
Source of genome sequence
The complete genome sequence of C. diphtheriae was downloaded from NCBI ftp site [22], and the DtxR-binding sites identified by experimental methods were collected from literature [6, 10, 25–27].
Prediction of DtxR-binding sites
DtxR-binding site recognition profile was calculated by positional Shannon relative entropy method [23, 24]. The positional relative entropy Q i at position i in a binding site is defined as

where b refers to each of the possible base (A, T, G, C), f b,i is observed frequency of each base at position i and q b is the frequency of base b in the genome sequence. The contribution of each base to the positional Shannon's relative entropy is calculated by multiplying positional frequency of each base with positional relative entropy. The binding site profile thus generated was used to scan upstream sequences of all the genes of the Corynebacterium diphtheriae genome. The score of each site is calculated as the sum of the respective positional Shannon relative entropy of each of the four possible bases. A maximally scoring site is selected from the upstream sequence of each gene. The lowest score among the input binding sites is considered as cut-off score. The sites scoring higher than the cut-off value are reported as potential binding sites conforming to the consensus sequence.
Prediction of operons
Co-directionally transcribed genes, downstream to the predicted binding site were selected as potential co-regulated genes (operons) according to one of the following criteria (a) Co-directionally transcribed orthologous gene pairs, conserved in at least 4 genomes; (b) genes belong to the same cluster of orthologous gene function category and the intergenic distance is less than 200 base pairs; (c) the first three letters in gene names are identical (gene names for putative genes were assigned from COG database); (d) intergenic distance is less than 90 base pairs [24].
Functional assignment of genes
The function of predicted genes was inferred using the RPS-BLAST search against conserved domain database [12]. These genes were further classified according to their function.
Expression and purification of IdeR
The iron-dependent regulator IdeR from M. tuberculosis was expressed from a recombinant pRSET vector containing the IdeR gene fused to a six His affinity tag (P. Chakhiyar unpublished). The expressed protein was first purified using Ni-NTA Metal Chelate Affinity chromatography; later it was desalted and concentrated using Centricon Ultra filtration device. The concentration of the recombinant protein was estimated using Bradford method.
Electrophoretic mobility shift assay
Double-stranded oligonucleotides containing the predicted binding motif (19 bp long) were end labeled with T4 polynucleotide kinase and [γ32P]-ATP and were incubated with the recombinant purified IdeR protein in a binding reaction mixture. The binding reaction mixture (20-μl total volume) contain the DNA-binding buffer (20 mM Tris-HCl [pH 8.0], 2 mM DTT, 50 mM NaCl, 5 mM MgCl2, 50% glycerol, 5 μg of bovine serum albumin per ml), 10 μg of poly(dI-dC) per ml (for nonspecific binding) and 200 μM MnCl2. The reaction mixture was incubated at room temperature for 30 min. Approximately 2 μl of the tracking dye (50% sucrose, 0.6% bromophenol blue) was added to the reaction mixture at the end of incubation and was loaded onto 7% polyacrylamide gel containing 150 μM MnCl2 in 1 × Tris-borate-EDTA buffer. The gel was electrophoresed at 200 V for 2 hours. Subsequently the gel was dried and exposed to Fuji Storage Phosphor Image Plates for 16 hours. The image plates were subsequently scanned in Fuji Storage Phosphor Imaging workstation.
Abbreviations
- DtxR:
-
Diphtheria toxin repressor
- IdeR:
-
Iron-dependent regulator
- Dps:
-
DNA-binding protein from starved cells
- RPS-BLAST:
-
Reversed Position Specific – Basic Local Alignment Search Tool
- EMSA:
-
Electrophoretic Mobility Shift Assay
References
Castagnetto JM, Hennessy SW, Roberts VA, Getzoff ED, Tainer JA, Pique ME: MDB: the Metalloprotein Database and Browser at The Scripps Research Institute. Nucleic Acids Res. 2002, 30: 379-382. 10.1093/nar/30.1.379.
Urbanski NK, Beresewicz A: Generation of *OH initiated by interaction of Fe2+ and Cu+ with dioxygen; comparison with the Fenton chemistry. Acta Biochim Pol. 2000, 47: 951-962.
Tao X, Schiering N, Zeng HY, Ringe D, Murphy JR: Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol. 1994, 14: 191-197.
Russo TA, Carlino UB, Johnson JR: Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun. 2001, 69: 6209-6216. 10.1128/IAI.69.10.6209-6216.2001.
Register KB, Ducey TF, Brockmeier SL, Dyer DW: Reduced virulence of a Bordetella bronchiseptica siderophore mutant in neonatal swine. Infect Immun. 2001, 69: 2137-2143. 10.1128/IAI.69.4.2137-2143.2001.
Qian Y, Lee JH, Holmes RK: Identification of a DtxR-regulated operon that is essential for siderophore-dependent iron uptake in Corynebacterium diphtheriae. J Bacteriol. 2002, 184: 4846-4856. 10.1128/JB.184.17.4846-4856.2002.
Kunkle CA, Schmitt MP: Analysis of the Corynebacterium diphtheriae DtxR Regulon: Identification of a putative siderophore synthesis and transport system that is similar to the Yersinia high-pathogenicity island-encoded yersiniabactin synthesis and uptake system. J Bacteriol. 2003, 185: 6826-6840. 10.1128/JB.185.23.6826-6840.2003.
Oram DM, Avdalovic A, Holmes RK: Construction and characterization of transposon insertion mutations in Corynebacterium diphtheriae that affect expression of the diphtheria toxin repressor (DtxR). J Bacteriol. 2002, 184: 5723-5732. 10.1128/JB.184.20.5723-5732.2002.
Cerdeno-Tarraga AM, Efstratiou A, Dover LG, Holden MT, Pallen M, Bentley SD, Besra GS, Churcher C, James KD, De Zoysa A, Chillingworth T, Cronin A, Dowd L, Feltwell T, Hamlin N, Holroyd S, Jagels K, Moule S, Quail MA, Rabbinowitsch E, Rutherford KM, Thomson NR, Unwin L, Whitehead S, Barrell BG, Parkhill J: The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res. 2003, 31: 6516-6523. 10.1093/nar/gkg874.
Lee JH, Wang T, Ault K, Liu J, Schmitt MP, Holmes RK: Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect Immun. 1997, 65: 4273-4280.
Feese MD, Ingason BP, Goranson-Siekierke J, Holmes RK, Hol WG: Crystal structure of the iron-dependent regulator from Mycobacterium tuberculosis at 2.0-A resolution reveals the Src homology domain 3-like fold and metal binding function of the third domain. J Biol Chem. 2001, 276: 5959-66. 10.1074/jbc.M007531200.
Marchler-Bauer A, Anderson JB, DeWeese-Scott C, Fedorova ND, Geer LY, He S, Hurwitz DI, Jackson JD, Jacobs AR, Lanczycki CJ, Liebert CA, Liu C, Madej T, Marchler GH, Mazumder R, Nikolskaya AN, Panchenko AR, Rao BS, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Vasudevan S, Wang Y, Yamashita RA, Yin JJ, Bryant SH: CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 2003, 31: 383-387. 10.1093/nar/gkg087.
de Lorenzo V, Neilands JB: Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J Bacteriol. 1986, 167: 350-355.
Martinez A, Kolter R: Protection of DNA during oxidative stress by the non specific DNA-binding protein Dps. J Bacteriol . 1997, 179: 5188-5194.
Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND: Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem. 2002, 277: 27689-27696. 10.1074/jbc.M202094200.
Zaika EI, Perlow RA, Matz E, Broyde S, Gilboa R, Grollman AP, Zharkov DO: Substrate discrimination by formamidopyrimidine-DNA glycosylase: a mutational analysis. J Biol Chem. 2004, 279: 4849-4861. 10.1074/jbc.M310262200.
Gaudu P, Weiss B: Flavodoxin mutants of Escherichia coli K-12. J Bacteriol. 2000, 182: 1788-1793. 10.1128/JB.182.7.1788-1793.2000.
Outten FW, Wood MJ, Munoz FM, Storz G: The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. 2003, 278: 45713-45719. 10.1074/jbc.M308004200.
Ton-That H, Schneewind O: Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol. 2003, 50: 1429-1438. 10.1046/j.1365-2958.2003.03782.x.
Suh JW, Boylan SA, Oh SH, Price CW: Genetic and transcriptional organization of the Bacillus subtilis spc-alpha region. Gene. 1996, 169: 17-23. 10.1016/0378-1119(95)00757-1.
Jannick DB, Henrik N, Gunnar VH, Søren B: Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004, 340: 783-795. 10.1016/j.jmb.2004.05.028.
NCBI FTP site.ftp://ftp.ncbi.nih.gov/genomes/Bacteria/Corynebacterium_diphtheriae
Shannon CE: A mathematical theory of communication. Bell System Technical Journal. 1948, 379-423. 623–656
Yellaboina S, Seshadri J, Kumar MS, Ranjan A: PredictRegulon: A webserver for the prediction of the regulatory protein binding sites and operons in prokaryote genomes. Nucleic Acids Res . 2004, 32: W318-W320.
Tao X, Murphy JR: Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem. 1992, 267: 21761-21764.
Schmitt MP, Holmes RK: Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J Bacteriol. 1994, 176: 1141-1149.
Schmitt MP: Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect Immun. 1997, 65: 4634-4641.
Acknowledgements
This work is partially supported by CSIR NMITLI Grant to AR. SR is supported by CSIR NMITLI Grant. YS and PC is supported by CSIR Research Fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
SY: carried out the computation, data analysis, and manuscript preparation. SR: Carried out the EMSA and drafted the manuscript. PC: provided the cloned IdeR construct, drafted the manuscript. SH: Manuscript preparation and coordination. AR: Design of the study and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yellaboina, S., Ranjan, S., Chakhaiyar, P. et al. Prediction of DtxR regulon: Identification of binding sites and operons controlled by Diphtheria toxin repressor in Corynebacterium diphtheriae. BMC Microbiol 4, 38 (2004). https://doi.org/10.1186/1471-2180-4-38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-4-38