Abstract
Background
While much recent research has expanded our understanding of the molecular interactions between aphids and their host plants, it is lacking for the soybean aphid, Aphis glycines. Since its North American invasion, A. glycines has become one of the most damaging insect pests on this important crop. Five soybean genes for host plant resistance to A. glycines have been identified, but populations of A. glycines have already adapted to overcome these resistance genes. Understanding the molecular interactions between resistant soybean and A. glycines can provide clues to its adaptation mechanisms. Here, we used RNA-Sequencing to compare and contrast A. glycines gene expression when fed resistant (Rag1) and susceptible soybean.
Results
Combining results from a previous A. glycines transcriptome, we generated 64,860 high quality transcripts, totaling 41,151,086 bases. Statistical analysis revealed 914 genes with significant differential expression. Most genes with higher expression in A. glycines on resistant plants (N = 352) were related to stress and detoxification such as cytochrome P450s, glutathione-S-transferases, carboxyesterases, and ABC transporters. A total of 562 genes showed lower transcript abundance in A. glycines on resistant plants. From our extensive transcriptome data, we also identified genes encoding for putative salivary effector proteins (N = 73). Among these, 6 effector genes have lower transcript abundance in A. glycines feeding on resistant soybean.
Conclusions
Overall, A. glycines exhibited a pattern typical of xenobiotic challenge, thereby validating antibiosis in Rag1, presumably mediated through toxic secondary metabolites. Additionally, this study identified many A. glycines genes and gene families at the forefront of its molecular interaction with soybean. Further investigation of these genes in other biotypes may reveal adaptation mechanisms to resistant plants.
Similar content being viewed by others
Background
The ability of plants to defend against insect feeding has long been a research focus to understand adaptation and co-evolution [1]. Sometimes referred to as a classic evolutionary arms race, these naturally evolved systems are often exploited in crop plants that offer resistance to insect pests as a way to prevent damage and protect yield, i.e. host plant resistance (HPR) [2]. Many host-plant resistant cultivars target aphids because they are arguably the most insidious pests of agronomic and horticultural crops worldwide [3, 4]. Yet several aphid species have been able to overcome this resistance in the form of virulent biotypes, which threatens the utility and sustainability of aphid resistant varieties [4]. Research on the molecular interactions between aphids and their host plants will allow comparative approaches to both expand our understanding of co-evolution as well as improve the durability of plant resistance.
Induced plant defenses usually involve the production of plant secondary metabolites (PSMs) that are toxic to insects. In turn, most insects respond to PSMs by inducing an array of stress response proteins including enzymes for metabolic excretion [5]. The metabolic excretion of PSMs and other xenobiotics by insects tends to occur in three phases [5–7]. In phase I, the biological activity of the specific metabolite is reduced, with cytochrome P450s acting as principal enzymes [6]. In phase II, the by-products of phase I are conjugated with hydrophilic substances to increase water-solubility which facilitates their excretion [6]. Phase II enzymes include glutathione S-transferases (GSTs), carboxylesterases (COEs), and UDP-glucuronlytransferases (UGTs). Finally, in phase III, conjugated compounds are exported out of the cell by employing ATP-binding cassette (ABC) and other transmembrane transporters [6].
In addition to inducing xenobiotic metabolism genes, insect stress and defense responses can also involve important proteins such as heat-shock proteins, proteases (to evade plant protease inhibitors), and multicopper oxidases (e.g. laccase-1 to oxidize PSMs) [8, 9]. Transcriptional activity of insect xenobiotic stress response machinery is regulated by transcription factors (TFs) [10]. Previous evidence, though limited, suggests that aphids utilize diverse mechanisms, including detoxification and other defense pathways, to cope with PSMs and host-plant resistance [11].
Another important factor in aphid-plant interactions are effectors [12–14]. Primarily, effectors are proteins or small molecules present in aphid saliva which modify the structure and function of a plant cell and can ultimately promote insect virulence and survival and/or trigger plant defense response [12]. Although numerous candidate effector genes from various aphid species have been identified either at the transcriptomic or proteomic level, their expression dynamics during compatible (susceptible plant-virulent aphid) and incompatible (resistant plant-avirulent aphid) interactions remain largely unexplored.
The soybean aphid, Aphis glycines, is a major pest of soybean (Glycine max L.) in both its native Asia, as well as in North America where it is invasive [15, 16]. A. glycines can cause up to 58% yield loss in soybean and is estimated to have an annual economic loss of $3.6-4.9 billion on soybean production in North-America [17]. Additionally, the use of insecticides to manage A. glycines has led to a dramatic rise in input cost for soybean production [17, 18].
To minimize damage by A. glycines, host plant resistance has been a significant research focus as it can be effective, economical, and environmentally safe [2, 19]. To date, 5 major soybean genes (Rag1, Rag1c, Rag2, Rag3, and rag4) and 3 provisional genes (Rag1b, rag3, and Rag5) conferring resistance to A. glycines have been identified [20–23]. Among these, Rag1, known to exhibit both antibiosis (affecting insect biology leading to increased mortality or reduced longevity and reproduction) and antixenosis (affecting the insect behaviour leading to non-preference for feeding and colonization) has been commercialized since 2009 [19, 24]. However, prior to the commercial release of resistant varieties, virulent biotypes of A. glycines that can survive on HPR soybean had already been discovered. For A. glycines, 4 biotypes (named biotypes 1, 2, 3 and 4) are known so far, each with varying abilities to survive and reproduce on individual or pyramided Rag possessing soybean [25–27]. Thus, sustainable management of A. glycines using HPR remains a considerable challenge [19, 28].
A comprehensive understanding of the molecular interactions between soybean and A. glycines, at both the plant and insect level, can provide insights into the HPR mechanism and potential routes of virulence adaptation. Previous work has focused on the molecular responses of soybean to attack by A. glycines[29, 30], but corresponding studies on A. glycines are lacking. Here, we compared the molecular response of A. glycines when fed resistant (Rag1) or susceptible soybean using RNA-Sequencing (RNA-Seq) and determined whether the response was consistent with antibiosis or antixenosis. Previous electrical penetration graphs of A. glycines feeding behavior and soybean transcriptomic studies revealed that Rag1-mediated resistance is effective within the first few hours of infestation [29, 31]. A. glycines stylets reach sieve elements of susceptible and resistant plants in 6 h and 9 h, respectively [31], with phloem intake commencing afterwards. On resistant plants, A. glycines can be seen dispersing 16-24 h after infestation, most likely due to stress of plant toxins and/or non-preference. Effects of Rag1 on A. glycines culminate during 24-36 h after infestation when mortality ensues, either due to PSMs, starvation, or both. Therefore, in order to have a comprehensive understanding of effects of Rag1 resistance and to avoid capturing expression signatures occurring due to potential starvation stress, we focused on an early time point (12 h) in this interaction. Using RNA-Seq, we identified many A. glycines genes and gene families which are at molecular interface of its interaction with soybean and may play a critical role in virulence adaptation. Owing to high-throughput sequencing strategy, we also significantly enriched the existing transcriptomic resources for A. glycines, a non-model but important invasive aphid species, which will provide a foundation for future molecular studies in this insect.
Results
De novoassembly and annotation
RNA-Seq for A. glycines yielded a total of 122,008,352 high quality, 76-bases paired-end reads. We pooled RNA-Seq reads with a previous transcriptome (comprising of 19,293 transcripts from 454 pyrosequencing, see [32]) to improve coverage and quality of the assembly. Using the combined dataset, de novo assembly of A. glycines produced 64,860 high quality transcripts, totaling 41,151,086 bases. The length of the transcripts varied from 150-16,670 nucleotides with an average of 634 nucleotides (Figure 1A). The assembly’s N50 equaled 1,164 (length N for which 50% of all bases in the assembly are located in a transcript of length L < N), which is relatively high for a non-model organism.
A. glycines transcriptome annotation and comparative genomics. (A) Length distribution of 64,860 contigs in de novo assembly. Individual contigs are ordered on X-axis based on increasing size. (B) Ortholog hit ratio for transcripts calculated after BLASTx searches to genomes of A. pisum, B. mori, D. melanogaster, N. vitripennis, R. prolixus, and T. casteneum . (C) Venn diagram showing the number of transcript contigs with significant matches (unique and common) to genomes of A. pisum, D. melanogaster, R. prolixus, and T. casteneum. Significant matches (e value <1.0E-3) were calculated after pairwise comparisons (BLASTx) to each individual genome. (D) Comparison of GO term mappings distributions of A. glycines and A. pisum that belong to each of the three top-level GO categories (i.e. biological process, molecular function, and cellular component).
To determine the completeness of A. glycines transcriptome assembly, each transcript was compared to its putative ortholog in Acyrthosiphon pisum, Bombyx mori, Drosophila melanogaster, Nasonia vitripennis, Rhodnius prolixus, and Tribolium casteneum. Nearly, 50% of A. glycines transcripts (with a match) had an ortholog hit ratio (OHR) >0.5 (Figure 1B). Considering that an OHR value of 0 indicates a poor assembly and a value of 1 indicates a fully assembled transcriptome [33], our assembly for A. glycines seems to be fairly comprehensive.
Nearly 30% (19,154/64,860) of the A. glycines transcripts had one or more hits to protein sequences in the refseq_protein database at GenBank (complete file available upon request). The majority of the top blast hits for A. glycines transcripts were to insects (92.7%), whereas a small proportion showed top hits to bacteria, non-arthropod animals, plants, fungi and viruses (Additional file 1). As expected, 88.4% (of the 19,154 with a match) of top hits for A. glycines transcripts were to A. pisum, which has a well characterized genome [34]. A. glycines transcripts having no match may represent genes either with a novel function or whose function has not yet been designated. Interestingly, an InterProScan search revealed hits to protein signature domains for 18,832 out of the 45,706 transcripts without a match to the ref_seq protein database (41%), suggesting that many have homologs in other species that were undetected. Blastn searches with the unmatched A. glycines dataset (45,706 transcripts) revealed hits for 4,576 transcripts, with top hits to A. pisum (2,931/4,576) and Aphis craccivora (1,381/4,576). Nonetheless, a relatively high percentage of transcripts with ‘no match’ obtained in our study is not surprising as similar values are recorded for transcriptomes of other non-model insects [35–37].
Comparative genomics
Using pairwise blastx searches to protein databases for four model insects, significant matches for A. glycines transcripts (combined = 21,455/64,860) were obtained. A blastx search to the A. pisum database showed matches for highest number of A. glycines transcripts (n = 21,295) (Figure 1C). A majority of A. glycines transcripts (n = 11,318) had matches to all the searched databases. However, there were a substantial number of transcripts which uniquely matched to A. pisum (n = 6,324), whereas only a few uniquely matched to R. prolixus (n = 62), T. casteneum (n = 29), and D. melanogaster (n = 17) databases.
Functional annotation
Using blast2go, a total of 11,311 A. glycines transcripts were annotated. Observed gene ontology (GO) terms for each domain (biological process, molecular function and cellular component) were widely distributed into different categories. A comparison of percent mappings to each GO category between A. glycines and A. pisum revealed nearly identical distributions for both aphid species (Figure 1D). The majority of transcripts assigned to the ‘biological process’ domain were involved in cellular, regulatory, developmental, and reproductive activities, while the largest part of transcripts under ‘molecular function’ domain were predicted to have catalytic, binding and transporter functions. Through KEGG-based pathway analysis, A. glycines transcripts were assigned to one or more of 129 total pathways (Additional file 2). The majority of transcripts were assigned to pathways for metabolism of nitrogenous compounds (e.g. purine, pyrimidine, amino acids) and sugars (e.g. glucose). Interestingly, a total of 194 transcripts were assigned to 19 pathways for xenobiotic degradation and metabolism. Among them, transcripts encoding enzymes involved in metabolism (such as P450 enzymes) were the most abundant.
Differential gene expression in A. glycines feeding on Rag1-soybean
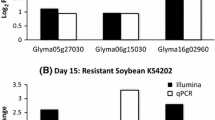
We obtained nearly 68 and 63 million RNA-Seq reads for A. glycines fed with resistant (possessing Rag1) and susceptible soybean, respectively (Table 1). For expression measurements, 77-87% of total reads mapped to reference database genes, with nearly all reads mapping uniquely. The read depth for reference database genes varied from 0 to 284,127, with an average of 264.9 reads per gene. Statistical analysis revealed 914 (out of 64,860 reference genes) differentially expressed genes (P <0.05) (Figure 2). The average expression level and read depth of all differentially expressed genes are provided in Additional file 3. A total of 362 and 552 up- and down-regulated genes, respectively, were found in A. glycines fed with Rag1 compared to those fed with the susceptible plant (Additional files 4 and 5). We chose 14 genes that spanned the range of differential expression and included several functional categories (based on RNA-Seq, see Additional file 6) to validate our statistical analysis with RNA-Seq. This comparison concurred with the expression pattern (either up- or down-regulated) and supported the accuracy and reliability of RNA-Seq in differential gene expression analysis (Figure 3). The GO enrichment analysis (Fisher test, agriGO) revealed 9 enriched ‘molecular function’ categories each among the up- and down-regulated genes (Additional file 7), which are detailed in following sections.
Gene expression changes in A. glycines due to Rag1 -soybean feeding. The expression (log2 fold change) of each gene between insects fed with resistant Rag1-soybean and those fed with susceptible plant is plotted against average expression level of each gene in both treatments. Fold change values for gene expression were considered significant if P values were <0.05. See materials and methods for details.
Up-regulation of genes related to xenobiotic metabolism and other stress responses
RNA-Seq analysis revealed several genes induced in A. glycines fed with Rag1-soybean potentially involved in all three phases of xenobiotic metabolism. For phase I, 13 genes related to cytochrome P450 (represented by 15 transcripts), were up-regulated. These putative P450 genes exhibited a higher transcript abundance ranging from 0.89-3.43 fold (log2 scale) in A. glycines on Rag1-soybean compared to those fed with the susceptible plant (Table 2). These genes were featured in 8 enriched gene ontology (GO) terms (Fisher test, FDR corrected P <0.05; Additional file 7). Nine out of 13 up-regulated putative P450 genes belonged to the CYP6 family from the CYP3 clan. For phase II, genes similar to GST, γ-glutamyltranspeptidase, COE, and sulfotransferase showed increased transcript abundance (Table 2). For phase III, transcript levels of 10 predicted ABC transporter genes, named AyABC1 to AyABC10, were higher in A. glycines fed with Rag1. Genes potentially involved in the cellular uptake (scavenger receptors AySR1-AySR4) and transfer (nose resistant to fluoxetine AyNRF1-AyNRF7) of xenobiotics were also up-regulated (Table 2). Other putative stress response genes, including 9 heat shock proteins (hsp) and 5 takeout (to) genes showed higher transcript levels (Additional file 8). Up-regulated hsp and to genes exhibited fold changes (log2 scale) that ranged from 1.76-3.24 and 1.00-1.92, respectively.
Differential expression of proteases, protease-inhibitors, and laccase-1 genes
RNA-Seq analysis revealed 6 protease-related genes having higher transcript abundance and 11 having lower transcript abundance in A. glycines on Rag1-soybean (Table 3, Additional file 4). All putative protease genes with higher transcript levels were most similar to serine proteases, and were named AySP1 to AySP6. The transcript levels for these genes exhibited an increase ranging from 0.88-1.78 fold (log2 scale). The putative protease genes with lower transcript levels in A. glycines feeding on Rag1-soybean included 7 genes similar to serine proteases and 4 genes encoding carboxypeptidases with reductions ranging from 1.45-4.06 and 0.93-2.62 fold (log2 scale), respectively. A. glycines feeding on Rag1-soybean also resulted in differential expression of 4 protease-inhibitor like genes (Table 3, Additional file 9). On sequence-based homology search (blastx), these protease-inhibitor like genes showed strong matches (e value ranged from 0.0 to 5.31244E-80; Additional file 4) to serine protease inhibitors (called serpins) of other insects, and were named as AySPI1 - AySPI4. Amongst these, AySPI1 and AySPI2 have higher transcript abundance whereas AySPI3 and AySPI4 have lower transcript abundance in A. glycines feeding on Rag1-soybean (Table 3). Four putative laccase-1 genes were also up-regulated in A. glycines on resistant plants (Table 3); transcript levels were in the range of 2.41-2.87 fold (log2 scale) greater in aphids on Rag1-soybean.
Suppression of putative salivary effector gene expression
As effectors play a central role in the molecular interaction between aphids and their host plants [14], we focused on genes that could encode for salivary effector proteins. Currently, there is little knowledge regarding A. glycines effectors; however using A. pisum effectors as queries (see methods) we found significant hits to our transcriptome, totaling 73 putative effectors (Additional file 10). However, only 6 putative effector transcripts were differentially expressed in A. glycines fed with Rag1 plant (Table 4). These 6 putative effectors showed 90-98% amino acid similarity to A. pisum effectors (Additional file 11, Table 4), and all were predicted to contain a secretion signal peptide at the N-terminal (Additional file 11). Our semi-quantitative PCR results confirmed effector expression in A. glycines salivary glands (Figure 4) as has been observed for their homologs in other aphids (references in Table 4). Interestingly, genes for these six effectors were down-regulated in A. glycines on Rag1-soybean compared to those feeding on susceptible plants; with reduction in transcript levels ranging between 0.85-4.50 fold (on log2 scale) (Table 4).
Gene expression of effectors in salivary glands of A. glycines . (A) A dissected out salivary gland from an A. glycines adult as viewed through a microscope. The principal salivary gland (PSG), the salivary duct (SD), and the accessory salivary gland (ASG) are indicated. (B) Results of semi quantitative PCR for expression analysis of effector genes in salivary gland and carcass (adult minus salivary gland and developing embryos) are shown. The PCR reactions were run for 35 cycles for all primer pairs except for AyDI2, where it was 40. The effector names and other details are provided in Table 4.
Differential gene expression in starved A. glycines
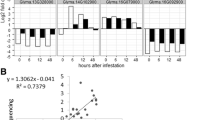
Rag1 leads to both antibiotic and antixenotic responses in A. glycines[24, 48], and the response seen in our study may also be related to starvation from the antixenotic response (i.e. non-preference). Due to the lack of a standardized and consistent artificial feeding (non-plant based) assay, we used starved aphids as a proxy to examine the molecular response that might be expected with antixenosis-induced starvation. Using qRT-PCR, we compared gene expression of the same 14 genes that encompassed a range of expression levels (Figure 3, Additional file 6) in aphids starved for 12 hr. We observed an overall pattern of reduced gene expression in starved aphids, and, in 13 out of 14 genes, the decrease was significant and expression was substantially less than what was observed when A. glycines was fed either Rag1 or susceptible soybean (average reduction ranged between 3.21-1,074.52 fold, Figure 5). For example, expression of disulphide isomerase like gene (a putative effector) was reduced by ~9.5 fold after starvation, but only ~1.5 fold after feeding on Rag1-soybean. In addition, P450s showed decreased expression in starved aphids, which would be expected in the absence of PSMs or other stress related to plant resistance.
Gene expression comparison among starved and fed A. glycines. Bars represent the relative mRNA levels of different genes in A. glycines using qRT-PCR. The mean (± S.E) expression level is represented for three biological replicates for A. glycines fed with resistant soybean (green bars), susceptible soybean (blue bars), and starved (grey bars). The elongation factor-la (AyEF1α) gene was used as an internal control for cDNA [77]. More details on genes and primer sequences are provided in Additional file 12. (* P < 0.05).
Discussion
In plant-aphid interactions, initial molecular recognition and signaling events are rapid and transient [49]. To identify the key genes involved, it is vital to focus on early time points, especially in an incompatible interaction (i.e. a resistant plant- avirulent insect) [49]. Our gene expression data was consistent with a rapid response by Rag1-soybean upon infestation with A. glycines[29]. Expression of genes typically involved in xenobiotic (PSMs) metabolism (e.g. P450s and GSTs) increased, whereas a few effectors showed decreased expression. These gene expression patterns were not similar to what was observed in starved aphids (Figure 5). Overall, our results indicated an active, rapid, and specific molecular xenobiotic stress response in A. glycines when fed resistant soybean and are consistent with earlier studies showing rapid responses in aphid-infested soybean and the presence of, yet unidentified, PSMs.
Feeding on resistant Rag1-soybean induces a xenobiotic stress response
PSMs, the defensive chemicals possessing direct toxicity to insect herbivores, are believed to occur as a complex mixture of inducers, substrates and inhibitors of insect xenobiotic response machinery [50]. We found that feeding A. glycines on resistant soybean resulted in the up-regulation of genes encoding for P450s, GSTs, COE, and ABC transporters (Table 2). This response is consistent with a typical xenobiotic challenge, resulting from the probable ingestion of PSMs present in Rag1-soybean, and supports the ‘antibiosis’ mode of HPR [48]. This increase was not due to starvation, as all P450’s were down regulated in starved A. gylcines (Figure 5). The involvement of some of these P450s and ABC transporters in other biological functions cannot be ruled out as these occur as large gene families known to perform multiple biological functions.
In Rag1-soybean, no specific PSM toxic to A. glycines has been reported due to a lack of metabolomics studies. However, a microarray-based study on Rag1-soybean responses to A. glycines feeding identified 17 differentially expressed genes for secondary metabolism [29]. Interestingly, 14 out of the 17 genes were related to the phenylpropanoid pathway (PPP) and encoded for homologs of chalcone synthase, isoflavone synthase, and a flavanone 3-hydroxylase-like protein. In plants, the PPP is a rich source of PSMs for defense against insect herbivores [51]. Soybean PPP produces PSMs like flavones, isoflavones, isoflavanones, and anthocyanins which can be potentially toxic to A. glycines[52]. Besides phenylpropanoids, phenolics appear to provide another layer of defense in Rag1-soybean, as indicated by the induction of laccase-1 genes in A. glycines (Table 3). Insect laccases are copper-containing enzymes which tend to detoxify plant phenolics through oxidation reactions [9].
Among 13 induced P450 genes in A. glycines feeding on Rag1-soybean, 9 belonged to the CYP6 family of CYP3 clan (Table 2). Members in this family have previously shown similar responses in other insects, and are specifically involved in metabolism of numerous PSMs. For example, CYP6B enzymes detoxify PSMs in Papilio polyxenes, P. multicaudatus, and Helicoverpa zea[50, 53, 54]. Interestingly, the induction of P450 and other xenobiotic metabolism genes revealed in current study occurred in an incompatible interaction (resistant plant-avirulent insect). In fact, the induction of xenobiotic response genes by PSM exposure is thought to be the first step leading to the eventual detoxification and virulence adaptation because mutations responsible for higher enzymatic potency toward a xenobiotic substrate are more likely to be selected if these occur in inducible genes (that overproduces the enzyme) rather than in constitutive genes [55]. Our findings strongly indicate a vital role for P450s in the coevolutionary history and apparent ‘arms race’ between A. glycines and soybean, and future investigation into role of xenobiotic response machinery may reveal adaptation mechanism in virulent biotypes.
Suppression of putative salivary effector gene expression
Among 73 putative A. glycines effectors identified in this study, 47 matched to A. pisum effectors with known function (Additional file 10). Based on homology, these effectors seem capable of performing diverse biological functions at the interface of aphid-plant interactions. Further, the down-regulation of 6 effectors in A. glycines fed with resistant plants (Table 4) seems to be specific, as expression of other putative infection-promoting effectors (e.g. peroxidase, cathepsin, serine carboxypeptidase) remain unchanged (Additional files 4 and 10). The mechanism of suppression after feeding on Rag1-soybean is unclear and may likely involve different possibilities. First, microRNAs (miRNAs) in Rag1-soybean may down-regulate A. glycines effector genes directly. Aphid resistant plants show differential expression of many conserved miRNA families upon aphid infestation compared to susceptible plants [56]. Furthermore, aphid tissues contain several plant miRNAs which are ingested during feeding on resistant plants [57]. The capability of microRNAs to perform cross-kingdom regulation (e.g. plant miRNAs regulating the expression of mammalian genes [58]) further supports a potential role of miRNAs regulating A. glycines gene expression.
Second, suppression of effector genes may be a by-product of the rapid induction of the xenobiotic metabolism machinery. Initially, to reach sieve element cells, aphid stylets follow an extracellular path surrounded by epidermal, mesodermal and parenchyma cells [14]. However, along the way, stylets puncture these cells (to assess their internal chemistry) and secrete saliva. Plants can then recognize aphid infestation and mount immediate defense responses in surface cells, even before stylets reach the sieve element cells. For example, after aphid infestation, VAT (for melon resistance to Aphis gossypii) and Mi-1.2 (for tomato resistance to Macrosiphum euphorbiae) based resistance is ubiquitous in various cell types [59, 60]. In fact, a broad, ubiquitous resistance expression is a typical feature of NBS-LRR family genes in plants [61]. There is a strong evidence that Rag1, also predicted to be a member of NBS-LRR family [29], mounts its defense ubiquitously in surface cellular layers [31]. Since the defense response is so rapid, shutting down salivary effector expression may lead to more efficient xenobiotic metabolism, resulting in a molecular trade-off. This may also explain the difference in magnitude of putative effector expression between aphids on resistant plants and starved aphids. Having no access to plants for a longer time period, starved aphids likely initiated an earlier and stronger suppression of gene expression (Figure 5). However, with a lack of a consistent artificial diet assay, it may be difficult to disentangle the effects of starvation.
Third, the resistance factors encountered in the sieve elements of Rag1-soybean may be responsible for decreased effector expression in A. glycines. This possibility is supported by the observation that aphid stylets stay for only 2.7 min in sieve elements of Rag1-soybean, as opposed to 18.9 min in susceptible plant [31] which ultimately results in reduced salivation. Otherwise, effector secretion is a continuous phenomenon for aphids on susceptible plants [62].
Protease and protease-inhibitor gene regulation against Rag1defence
As a part of defence against herbivores, plants deploy protease inhibitors which target insect digestive proteases and suppress enzymatic activities [63]. To combat this, herbivorous insects exhibit elevated levels of inhibitor-insensitive and/or reduced levels of inhibitor-sensitive proteases [64]. The observed differential expression of proteases in A. glycines feeding on the resistant plant (Table 3) occurred in response to the elevated levels of protease inhibitors in Rag1-soybean after aphid infestation [30]. However, modified A. glycines protease activity may have undesirable effects as it can be harmful to critical gut structures [65], in addition to the potential damage caused by plant proteinases [43, 44]. Thus, in order to protect itself from internal and external proteinases, it is likely that A. glycines differentially regulates protease inhibitors, as observed in this study (Table 3).
Conclusion
Soybean with Rag1 resistance induced the expression of genes encoding various stress response proteins such as P450s, GST, COE, ABC transporter, and HSP in A. glycines. Furthermore, feeding on Rag1-soybean resulted in the down-regulation of genes for putative effectors that were found in A. glycines salivary glands. The overall response in A. glycines due to Rag1 feeding resembled that of a characteristic xenobiotic challenge, which supported the ‘antibiosis’ mode of Rag1 HPR being mediated through toxic PSMs. The genes identified here will be prime candidates to investigate A. glycines biotype evolution.
Methods
Plant and insect source
Two soybean lines, LD05-16060 (carrying Rag1 resistance to biotype 1 of A. glycines) and SD01-76R (the susceptible near-isoline of LD05-16060) were used. LD05-16060 was developed through backcrossing twice the variety Dowling (Rag1) [66] into the background of SD01-76R. The pedigree of LD05-16060 is SD01-76R(2) x Dowling x Loda.
To prepare cDNA libraries for RNA-Seq and to perform subsequent qRT-PCR validation, A. glycines were obtained from a biotype 1 laboratory colony that originated from insects collected from Urbana, IL (40° 06′ N, 88° 12′ W) in 2000 [67]. These aphids are defined as being avirulent to all known Rag genes. At the Ohio Agricultural Research and Development Center (OARDC) Wooster, OH, a laboratory population of these insects is maintained on susceptible soybean seedlings (SD01-76R) in a rearing room at 23-25°C and a photoperiod of 14:10 h (Light:Dark). All A. glycines in this OARDC colony are descended from 1 single founding female and represent 1 clonal lineage to limit variation from multiple genetic backgrounds.
A. glycines feeding on resistant (Rag1) and susceptible soybean
To obtain newborn nymphs, A. glycines adults (apterate females) were transferred (using a camel hair brush) and allowed to feed on detached trifoliate soybean leaves (SD01-76R) in a petri dish [68]. After 2 h, the newly hatched nymphs of A. glycines were delicately transferred onto intact first trifoliate leaves of LD05-16060 and SD01-76R whole plants, grown in separate pots. Following the transfer, infested leaves were isolated with a small snap cage to restrict the insect movement. Snap cages contained holes covered with wire mesh to allow for proper ventilation and maintenance of optimum growth conditions. Nymphs were allowed to feed on respective plants for 12 h. Following the feeding, insects were collected in a 1.5 ml eppendorf tube and were immediately frozen at -80°C. Nymphs fed with 3 separate plants of identical soybean line (LD05-16060 or SD01-76R) were pooled to constitute one biological replicate of each treatment. Nearly 60-70 nymphs were collected for each replication and there were three biological replications for each treatment.
RNA extraction and RNA-Seq libraries preparation
Insect samples were processed for total RNA extraction using PureLink® RNA Mini Kit (Life Technologies Corporation, Carlsbad, CA, US), following the manufacturer’s protocol. To remove DNA contamination, samples were treated with PureLink® DNase (Life Technologies Corporation, Carlsbad, CA, US). RNA quality was checked using a Nanodrop 2000c (Thermo Scientific, Hudson, NH, US) and an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, US). The cDNA libraries for RNA-Seq were prepared using the TruSeq RNA Sample Preparation Kit (Illumina Inc., San Diego, CA, US), following the manufacturer’s protocol. Briefly, 4 μg of total RNA for each sample was used to purify and fragment mRNA (library insert fragmentation at 94°C for 8 min to give an insert of 155 bp; range 120-210 bp), followed by first and second strand cDNA synthesis. Then, a series of steps including end-repair (to convert the overhangs resulting from fragmentation into blunt ends), adenylation of 3′ ends of the blunt fragments (to prevent them from ligating to one another during the adapter ligation reaction), ligation of adapters to the ends of double stranded cDNA, and PCR amplification to enrich DNA fragments with adapters were performed. Unique adapter sequences were included for each of the three biological replicates from each treatment. The high quality of the libraries was confirmed using a high sensitivity DNA chip on Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, US). The libraries for 3 biological replicates of each treatment were pooled together, and the pooled sample from each treatment was sequenced in two lanes of a Genome Analyzer II flow cell (Illumina Inc., San Diego, CA, US). The paired-end sequencing was performed at Molecular and Cellular Imaging Center (MCIC), OARDC, Wooster, OH, USA.
Raw sequencing data processing
For sample-wise allocation of the sequencing data, the raw reads from each lane of flow cell were demultiplexed using the respective index sequence. Initial processing of sequencing data was performed using MCIC galaxy tools available at http://www.oardc.ohio-state.edu/mcic/. Briefly, fastq quality scores of reads were converted from ‘Illumina1.5′ type to ‘Sanger’ type using FASTQ Groomer (version 1.0.4) [69]. The adapter sequences were removed from sequencing reads using ‘cutadapt’ (version 0.9.5.a) tool [70]. The quality check on sequencing reads was performed using ‘fastqc’ tool (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Further, reads were trimmed using ‘Trim the reads by quality’ (version 1.2.2) tool (Phred quality cutoff of 20 and minimum read length of 40 nucleotides). All sequence data were deposited in the GenBank under the BioProject accession PRJNA231526.
De novoassembly construction and functional annotation
The de novo assembly and subsequent gene expression analyses were performed using CLC Genomics Workbench version 6.02 (CLC Bio, Aarhus, Denmark). In addition to the Illumina RNA-Seq data from this study, previously published Roche 454 cDNA transcripts [32] were used as input for assembly. Assembly proceeded using the following parameters: word size of 24, bubble size of 50, and minimum contig size of 150 bases. The A. glycines transcriptome was annotated using Blast2GO, which implemented BLASTx searches (e value <1.0E-3) between all A. glycines contigs and the NCBI Reference Sequence database (Refseq_protein) [71]. Following the mapping step, gene ontology (GO) terms with e value <1.0E-6, annotation cut-off >55, and GO weight >5 were used for annotation. To categorize the GO terms into different GO categories, CateGOrizer, was used, along with the ‘GO_slim’ classification [72]. The GO categories for A. glycines were compared to those from A. pisum, available at http://www.b2gfar.org/showspecies?species=7029. The A. glycines contigs that showed no match to the Refseq_protein database were searched using BLASTn (e value <1.0E-3) for hits to the non-redundant nucleotide (nt) database at NCBI. Functionally enriched GO terms in the differentially expressed gene dataset (see below) were identified through the singular enrichment analysis (Fisher test; Yekutieli FDR corrected P <0.05) in agriGO (http://bioinfo.cau.edu.cn/agriGO/) [73]. To find the pathways in which putative proteins of A. glycines transcriptome are involved, analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) was performed using Blast2GO [74]. For comparative genomics, pairwise BLASTx searches (E value <1.0E-3) between A. glycines contig sequences and genomes of model insect species (A. pisum, B. mori, D. melanogaster, N. vitripennis, R. prolixus, T. casteneum) were performed. Results of these blastx searches were also used to calculate the ortholog hit ratio (at OARDC MCIC galaxy, http://www.oardc.ohio-state.edu/mcic/).
Differential gene expression analysis
To obtain the gene expression values, reads from each sample replicate were mapped to the genes (i.e. assembly contigs) with the default mapping parameters {minimum similarity fraction = 0.8 and minimum length fraction (long reads) = 0.9} [75]. Statistical comparison of expression values from both treatments was conducted using bootstrapped receiver operating characteristic (bROC) algorithm, available as an integrated plug-in in CLC Bio genomics workbench. To avoid problems with infinite values, the expression values were transformed as log2(E + 1); where E is the original expression value. The expression data was normalized using median of M-values (MMV) method. While calculating fold change for gene expression changes, expression values for A. glycines fed with susceptible soybean (SD01-76R) were taken as reference. Fold change values for gene expression were considered significant if P values were <0.05.
qRT-PCR on starved A. glycinesand for validation of RNA-Seq data
To starve A. glycines, newly hatched nymphs were placed in petri dishes containing only moist filter paper for 12 h. For RNA-Seq data validation, insect samples were collected as described above for RNA-Seq library preparation. Samples were processed for RNA extraction and first strand cDNA synthesis as described previously [76]. Specific primers for each gene were designed using Beacon Designer version 7.0 (Premier Biosoft, Palo Alto, CA) (Additional file 12). Due to their consistent expression, TBP and EF1a were used as internal controls, using previously described conditions [77]. There were 3 biological and 2 technical replications for each gene validated. Relative expression level and fold change were determined using comparative Ct method (2-∆∆Ct) [78]. Statistical analysis was performed using t-test through MeV package, version 4.9 available at http://www.tm4.org.
Identification and validation of salivary effector genes
Initially, to identify A. glycines salivary effector transcripts, A. pisum effector protein sequences [13] were used as the query in a tblastn search (top hit e value cut-off: 1E-20; bit score cut-off: 250) among the differentially expressed A. glycines genes. Identity of putative A. glycines effector transcripts was confirmed by blastx search at NCBI-GenBank. Subsequently, identified transcripts were filtered out based on 5 criterions to validate their salivary effector nature: 1) minimum 90% similarity of encoded proteins to A. pisum effectors; 2) expression in salivary glands as revealed by semi-quantitative PCR (method is described below); 3) presence of secretion signal peptide in encoded proteins as revealed by signalP version 4.1. [79]; 4) absence of transmembrane domain in encoded proteins as revealed by TMHMM server version 2.0 [80]; and 5) presence of signature domains in encoded proteins as revealed by an interProScan search which were inspected manually (e.g. contig_7391’s coding region contained signature motifs for peptidaseM1 (IPR001930) and metalloprotease (PTHR11533) activities). Due to the minuscule size of nymphs, adult A. glycines were used to dissect out salivary glands (10 days old) in phosphate-buffered saline (pH 8.0). Both salivary gland and carcass (adult minus salivary glands and developing embryos) samples (50 individuals each) were processed for RNA extraction, DNA-ase treatment and first strand cDNA synthesis as described previously [77]. The primer sequences are given in Additional file 12. Each RT-PCR reaction was performed with 1 μl of cDNA (100 ng /μl), 0.5 μM of each primer, and 10 μl of PCR master mix (from Promega) in a 20-μl total volume. The PCR amplifications were done with the following cycling conditions: one cycle at 95°C (3 min), followed by 35-40 cycles of denaturation at 95°C (30 s), annealing and extension at 55°C for 30 s.
Availability of supporting data
All sequence data were deposited in the GenBank under BioProject accession PRJNA231526. Additionally, the nucleotide sequence for each contig described in this study is provided in Additional file 9.
Abbreviations
- HPR:
-
Host plant resistance
- PSM:
-
Plant secondary metabolite.
References
Tilmon KJ: Specialization, Speciation, and Radiation: the Evolutionary Biology of Herbivorous Insects. 2008, Berkeley, CA: University of California Press, 360-
Smith CM: Plant Resistance to Arthropods: Molecular and Conventional Approaches. 2005, AA Dordrecht, The Netherlands: Springer, 423-
Van Emden HF, Harrington R: Aphids as Crop Pests/edited by Helmut F. van Emden and Richard Harrington. 2007, Oxfordshire, UK: CABI Publishing
Smith CM, Chuang W: Plant resistance to aphid feeding: behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag Sci. 2014, 70: 528-540. 10.1002/ps.3689.
Li X, Schuler MA, Berenbaum MR: Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007, 52: 231-253. 10.1146/annurev.ento.51.110104.151104.
Xu C, Li CY, Kong AT: Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005, 28 (3): 249-268. 10.1007/BF02977789.
Misra JR, Horner MA, Lam G, Thummel CS: Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25 (17): 1796-1806. 10.1101/gad.17280911.
Jongsma M, Beekwilder J: Co-evolution of insect proteases and plant protease inhibitors. Curr Protein Pept Sci . 2011, 12 (5): 437-447. 10.2174/138920311796391115.
Prasain K, Nguyen TD, Gorman MJ, Barrigan LM, Peng Z, Kanost MR, Syed LU, Li J, Zhu KY, Hua DH: Redox potentials, laccase oxidation, and antilarval activities of substituted phenols. Bioorg Med Chem. 2012, 20 (5): 1679-1689. 10.1016/j.bmc.2012.01.021.
King-Jones K, Horner MA, Lam G, Thummel CS: The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006, 4 (1): 37-48. 10.1016/j.cmet.2006.06.006.
Cai Q, Han Y, Cao Y, Hu Y, Zhao X, Bi J: Detoxification of gramine by the cereal aphid Sitobion avenae. J Chem Ecol. 2009, 35 (3): 320-325. 10.1007/s10886-009-9603-y.
Hogenhout SA, Bos JI: Effector proteins that modulate plant–insect interactions. Curr Opin Plant Biol. 2011, 14 (4): 422-428. 10.1016/j.pbi.2011.05.003.
Carolan JC, Caragea D, Reardon KT, Mutti NS, Dittmer N, Pappan K, Cui F, Castaneto M, Poulain J, Dossat C: Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res. 2011, 10 (4): 1505-1518. 10.1021/pr100881q.
Elzinga DA, Jander G: The role of protein effectors in plant–aphid interactions. Curr Opin Plant Biol. 2013, 16 (4): 451-456. 10.1016/j.pbi.2013.06.018.
Tilmon K, Hodgson E, O’Neal M, Ragsdale D: Biology of the soybean aphid, Aphis glycines (Hemiptera: Aphididae) in the United States. J Int Pest Manag. 2011, 2 (2): A1-A7.
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N: Ecology and management of the soybean aphid in North America. Annu Rev Entomol. 2011, 56: 375-399. 10.1146/annurev-ento-120709-144755.
Hodgson E, McCornack B, Tilmon K, Knodel J: Management recommendations for soybean aphid (Hemiptera: Aphididae) in the United States. J Int Pest Manag. 2012, 3 (1): E1-E10.
Ragsdale DW, Voegtlin DJ, O’Neil RJ: Soybean aphid biology in North America. Ann Entomol Soc Am. 2004, 97 (2): 204-208. 10.1603/0013-8746(2004)097[0204:SABINA]2.0.CO;2.
Hesler LS, Chiozza MV, O’Neal ME, MacIntosh GC, Tilmon KJ, Chandrasena DI, Tinsley NA, Cianzio SR, Costamagna AC, Cullen EM: Performance and prospects of Rag genes for management of soybean aphid. Entomol Exp Appl. 2013, 147 (3): 201-206. 10.1111/eea.12073.
Hill CB, Li Y, Hartman GL: A single dominant gene for resistance to the soybean aphid in the soybean cultivar Dowling. Crop Sci. 2006, 46 (4): 1601-1605. 10.2135/cropsci2005.11-0421.
Hill CB, Li Y, Hartman GL: Soybean aphid resistance in soybean Jackson is controlled by a single dominant gene. Crop Sci. 2006, 46 (4): 1606-1608. 10.2135/cropsci2005.11-0438.
Mian MR, Kang S, Beil SE, Hammond RB: Genetic linkage mapping of the soybean aphid resistance gene in PI 243540. Theor Appl Genet. 2008, 117 (6): 955-962. 10.1007/s00122-008-0835-y.
Zhang G, Gu C, Wang D: A novel locus for soybean aphid resistance. Theor Appl Genet. 2010, 120 (6): 1183-1191. 10.1007/s00122-009-1245-5.
Diaz-Montano J, Reese JC, Schapaugh WT, Campbell LR: Characterization of antibiosis and antixenosis to the soybean aphid (Hemiptera: Aphididae) in several soybean genotypes. J Econ Entomol. 2006, 99 (5): 1884-1889. 10.1603/0022-0493-99.5.1884.
Kim K, Hill CB, Hartman GL, Mian M, Diers BW: Discovery of soybean aphid biotypes. Crop Sci. 2008, 48 (3): 923-928. 10.2135/cropsci2007.08.0447.
Hill CB, Crull L, Herman TK, Voegtlin DJ, Hartman GL: A new soybean aphid (Hemiptera: Aphididae) biotype identified. J Econ Entomol. 2010, 103 (2): 509-515. 10.1603/EC09179.
Alt J, Ryan-Mahmutagic M: Soybean aphid biotype 4 identified. Crop Sci. 2013, 53 (4): 1491-1495. 10.2135/cropsci2012.11.0672.
Bansal R, Jun T, Mian M, Michel AP: Developing Host-Plant Resistance for Hemipteran Soybean Pests: Lessons from Soybean Aphid and Stink Bugs. Soybean - Pest Resistance. Edited by: El-Shemy HA. 2013, Rijeka, Croatia: INTECH Publishing, 19-46.
Li Y, Zou J, Li M, Bilgin DD, Vodkin LO, Hartman GL, Clough SJ: Soybean defense responses to the soybean aphid. New Phytol. 2008, 179 (1): 185-195. 10.1111/j.1469-8137.2008.02443.x.
Studham ME, MacIntosh GC: Multiple phytohormone signals control the transcriptional response to soybean aphid infestation in susceptible and resistant soybean plants. Mol Plant-Microbe Interact. 2013, 26 (1): 116-129. 10.1094/MPMI-05-12-0124-FI.
Diaz-Montano J, Reese JC, Louis J, Campbell LR, Schapaugh WT: Feeding behavior by the soybean aphid (Hemiptera: Aphididae) on resistant and susceptible soybean genotypes. J Econ Entomol. 2007, 100 (3): 984-989. 10.1603/0022-0493(2007)100[984:FBBTSA]2.0.CO;2.
Bai X, Zhang W, Orantes L, Jun T, Mittapalli O, Mian MR, Michel AP: Combining next-generation sequencing strategies for rapid molecular resource development from an invasive aphid species. Aphis glycines PLoS One. 2010, 5 (6): e11370-10.1371/journal.pone.0011370.
O’Neil ST, Dzurisin JD, Carmichael RD, Lobo NF, Emrich SJ, Hellmann JJ: Population-level transcriptome sequencing of nonmodel organisms Erynnis propertius and Papilio zelicaon. BMC Genomics. 2010, 11: 310-2164-11-310-
Consortium IAG: Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8 (2): e1000313-10.1371/journal.pbio.1000313.
Mittapalli O, Bai X, Mamidala P, Rajarapu SP, Bonello P, Herms DA: Tissue-specific transcriptomics of the exotic invasive insect pest emerald ash borer (Agrilus planipennis). PLoS One. 2010, 5 (10): e13708-10.1371/journal.pone.0013708.
Chen Y, Cassone BJ, Bai X, Redinbaugh MG, Michel AP: Transcriptome of the plant virus vector Graminella nigrifrons, and the molecular interactions of maize fine streak rhabdovirus transmission. PLoS One. 2012, 7 (7): e40613-10.1371/journal.pone.0040613.
Bai X, Mamidala P, Rajarapu SP, Jones SC, Mittapalli O: Transcriptomics of the bed bug (Cimex lectularius). PLoS One. 2011, 6 (1): e16336-10.1371/journal.pone.0016336.
Nicholson SJ, Hartson SD, Puterka GJ: Proteomic analysis of secreted saliva from Russian Wheat Aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J Proteome. 2012, 75 (7): 2252-2268. 10.1016/j.jprot.2012.01.031.
Carolan JC, Fitzroy CI, Ashton PD, Douglas AE, Wilkinson TL: The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics. 2009, 9 (9): 2457-2467. 10.1002/pmic.200800692.
Atamian HS, Chaudhary R, Cin VD, Bao E, Girke T, Kaloshian I: In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant-Microbe Interact. 2013, 26 (1): 67-74. 10.1094/MPMI-06-12-0144-FI.
Rao SA, Carolan JC, Wilkinson TL: Proteomic profiling of cereal aphid saliva reveals both ubiquitous and adaptive secreted proteins. PLoS One. 2013, 8 (2): e57413-10.1371/journal.pone.0057413.
Cui F, Michael Smith C, Reese J, Edwards O, Reeck G: Polymorphisms in salivary gland transcripts of Russian wheat aphid biotypes 1 and 2. Insect Science. 2012, 19 (4): 429-440. 10.1111/j.1744-7917.2011.01487.x.
Pechan T, Ye L, Chang Y, Mitra A, Lin L, Davis FM, Williams WP, Luthe DS: A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other Lepidoptera. The Plant Cell Online. 2000, 12 (7): 1031-1040. 10.1105/tpc.12.7.1031.
Mohan S, Ma PW, Williams WP, Luthe DS: A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLoS One. 2008, 3 (3): e1786-10.1371/journal.pone.0001786.
Will T, Tjallingii WF, Thönnessen A, van Bel AJ: Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci. 2007, 104 (25): 10536-10541. 10.1073/pnas.0703535104.
Will T, Kornemann SR, Furch AC, Tjallingii WF, van Bel AJ: Aphid watery saliva counteracts sieve-tube occlusion: a universal phenomenon?. J Exp Biol. 2009, 212 (20): 3305-3312. 10.1242/jeb.028514.
Darby NJ, Penka E, Vincentelli R: The multi-domain structure of protein disulfide isomerase is essential for high catalytic efficiency. J Mol Biol. 1998, 276 (1): 239-247. 10.1006/jmbi.1997.1504.
Li Y, Hill CB, Hartman GL: Effect of three resistant soybean genotypes on the fecundity, mortality, and maturation of soybean aphid (Homoptera: Aphididae). J Econ Entomol. 2004, 97 (3): 1106-1111. 10.1603/0022-0493(2004)097[1106:EOTRSG]2.0.CO;2.
Thompson GA, Goggin FL: Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot. 2006, 57 (4): 755-766. 10.1093/jxb/erj135.
Feyereisen R, Feyereisen R: Insect CYP Genes and P450 Enzymes. Insect Molecular Biology And Biochemistry. Edited by: Gilbert LH. 2012, London: Academic Press/Elsevier, 236-315.
Dixon RA, Achnine L, Kota P, Liu C, Reddy M, Wang L: The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol. 2002, 3 (5): 371-390. 10.1046/j.1364-3703.2002.00131.x.
Zabala G, Zou J, Tuteja J, Gonzalez D, Clough S, Vodkin L: Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to pseudomonas syringae infection. BMC Plant Biol. 2006, 6 (1): 26-10.1186/1471-2229-6-26.
Mao W, Berhow MA, Zangerl AR, Mcgovern J, Berenbaum MR: Cytochrome P450-mediated metabolism of xanthotoxin by Papilio multicaudatus. J Chem Ecol. 2006, 32 (3): 523-536. 10.1007/s10886-005-9018-3.
Puinean AM, Foster SP, Oliphant L, Denholm I, Field LM, Millar NS, Williamson MS, Bass C: Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6 (6): e1000999-10.1371/journal.pgen.1000999.
Despres L, David J, Gallet C: The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol. 2007, 22 (6): 298-307. 10.1016/j.tree.2007.02.010.
Sattar S, Song Y, Anstead JA, Sunkar R, Thompson GA: Cucumis melo microRNA expression profile during aphid herbivory in a resistant and susceptible interaction. Mol Plant-Microbe Interact. 2012, 25 (6): 839-848. 10.1094/MPMI-09-11-0252.
Sattar S, Addo-Quaye C, Song Y, Anstead JA, Sunkar R, Thompson GA: Expression of small RNA in Aphis gossypii and Its potential role in the resistance interaction with melon. PLoS One. 2012, 7 (11): e48579-10.1371/journal.pone.0048579.
Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai X: Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2011, 22 (1): 107-126.
Dogimont C, Bendahmane A, Chovelon V, Boissot N: Host plant resistance to aphids in cultivated crops: genetic and molecular bases, and interactions with aphid populations. C R Biol. 2010, 333 (6): 566-573.
Goggin FL, Jia L, Shah G, Hebert S, Williamson VM, Ullman DE: Heterologous expression of the Mi-1.2 gene from tomato confers resistance against nematodes but not aphids in eggplant. Mol Plant-Microbe Interact. 2006, 19 (4): 383-388. 10.1094/MPMI-19-0383.
McHale L, Tan X, Koehl P, Michelmore RW: Plant NBS-LRR proteins: adaptable guards. Genome Biol. 2006, 7 (4): 212-10.1186/gb-2006-7-4-212.
Giordanengo P, Brunissen L, Rusterucci C, Vincent C, Van Bel A, Dinant S, Girousse C, Faucher M, Bonnemain J: Compatible plant-aphid interactions: how aphids manipulate plant responses. C R Biol. 2010, 333 (6): 516-523.
Habib H, Fazili KM: Plant protease inhibitors: a defense strategy in plants. Biotechnol Mol Biol Rev. 2007, 2 (3): 68-85.
Bown DP, Wilkinson HS, Gatehouse JA: Differentially regulated inhibitor-sensitive and insensitive protease genes from the phytophagous insect pest, Helicoverpa armigera, are members of complex multigene families. Insect Biochem Mol Biol. 1997, 27 (7): 625-638. 10.1016/S0965-1748(97)00043-X.
Ge Z, Wan P, Cheng X, Zhang Y, Li G, Han Z, Bell J: Cloning and characterization of serpin-like genes from the striped rice stem borer. Chilo suppressalis Genome. 2013, 56 (6): 359-366.
Li Y, Hill CB, Carlson SR, Diers BW, Hartman GL: Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Mol Breed. 2007, 19 (1): 25-34.
Hill CB, Li Y, Hartman GL: Resistance to the soybean aphid in soybean germplasm. Crop Sci. 2004, 44 (1): 98-106. 10.2135/cropsci2004.0098.
Michel AP, Mian MR, Davila-Olivas NH, Cañas LA: Detached leaf and whole plant assays for soybean aphid resistance: differential responses among resistance sources and biotypes. J Econ Entomol. 2010, 103 (3): 949-957. 10.1603/EC09337.
Blankenberg D, Gordon A, Von Kuster G, Coraor N, Taylor J, Nekrutenko A, Galaxy Team: Manipulation of FASTQ data with Galaxy. Bioinformatics. 2010, 26 (14): 1783-1785. 10.1093/bioinformatics/btq281.
Martin M: Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet journal. 2011, 17 (1): 10-12. 10.14806/ej.17.1.200.
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M: Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005, 21 (18): 3674-3676. 10.1093/bioinformatics/bti610.
Zhi-Liang H, Bao J, Reecy J: CateGOrizer: a web-based program to batch analyze gene ontology classification categories. Online J Bioinformatics. 2008, 9: 108-112.
Du Z, Zhou X, Ling Y, Zhang Z, Su Z: agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38 (suppl 2): W64-W70.
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M: KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27 (1): 29-34. 10.1093/nar/27.1.29.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B: Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth. 2008, 5 (7): 621-628. 10.1038/nmeth.1226.
Bansal R, Mian M, Mittapalli O, Michel AP: Molecular characterization and expression analysis of soluble trehalase gene in Aphis glycines, a migratory pest of soybean. Bull Entomol Res. 2013, 1-10.
Bansal R, Mamidala P, Mian MR, Mittapalli O, Michel AP: Validation of reference genes for gene expression studies in Aphis glycines (Hemiptera: Aphididae). J Econ Entomol. 2012, 105 (4): 1432-1438. 10.1603/EC12095.
Schmittgen TD, Livak KJ: Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008, 3 (6): 1101-1108. 10.1038/nprot.2008.73.
Petersen TN, Brunak S, von Heijne G, Nielsen H: SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011, 8 (10): 785-786. 10.1038/nmeth.1701.
Krogh A, Larsson B, Von Heijne G, Sonnhammer EL: Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001, 305 (3): 567-580. 10.1006/jmbi.2000.4315.
Acknowledgements
We are grateful for the help provided by Lucinda Wallace in maintaining A. glycines colony, Asela Wijaratne and Saranga Wijaratne for assistance in transcriptome annotation. Priyanka Mittapelly assisted with qRT-PCR for the starved A. glycines. We are thankful to Matthew Rouhier and Peter Piermarini for assistance with microscopy. Soybean seeds were sent by David Ragsdale, and biotype 1 A. glycines were provided by Curt Hill. We are thankful to David Nelson for naming A. glycines P450 genes. Funding support was provided by OARDC, The Ohio State University, The Ohio Soybean Council and the North Central Soybean Research Program. Partial funding for open access was provided by The Ohio State University Open Access Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RB, MARM, OM, and APM conceived and designed the experiments, RB generated and analyzed the data, RB and AM interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12864_2014_6855_MOESM4_ESM.xlsx
Additional file 4: Annotation details for up-regulated and down-regulated genes in A. glycines fed with Rag1 -soybean. This file has two spreadsheets; one each for up-regulated and down-regulated gene dataset. (XLSX 96 KB)
12864_2014_6855_MOESM6_ESM.zip
Additional file 6: Visual representation of genes (indicated in red) chosen for qRT-PCR validation. Based on the expression level distribution, we chose 14 genes that supposedly represented the majority of differentially expressed genes. (ZIP 63 KB)
12864_2014_6855_MOESM11_ESM.zip
Additional file 11: Pairwise sequence alignment for salivary effector proteins of A. glycines and A. pisum. In the alignment of each effector protein, upper and lower lanes represent the A. glycines and A. pisum sequences respectively. At the N-terminal, putative secretion signal peptide regions are underlined. If the corresponding amino acid residues are identical, it is indicated by dots for A. pisum.(ZIP 912 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bansal, R., Mian, M., Mittapalli, O. et al. RNA-Seq reveals a xenobiotic stress response in the soybean aphid, Aphis glycines, when fed aphid-resistant soybean. BMC Genomics 15, 972 (2014). https://doi.org/10.1186/1471-2164-15-972
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-15-972