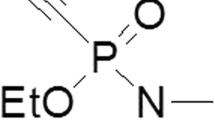
Abstract
Background
Most test systems for acetylcholinesterase activity (E.C.3.1.1.7.) are using toxic inhibitors (BW284c51 and iso-OMPA) to distinguish the enzyme from butyrylcholinesterase (E.C.3.1.1.8.) which occurs simultaneously in the cerebrospinal fluid. Applying Ellman's colorimetric method, we were looking for a non-toxic inhibitor to restrain butyrylcholinesterase activity. Based on results of previous in vitro studies bupivacaine emerged to be a suitable inhibitor.
Results
Pharmacokinetic investigations with purified cholinesterases have shown maximum inhibition of butyrylcholinesterase activity and minimal interference with acetylcholinesterase activity at bupivacaine final concentrations between 0.1 and 0.5 mmol/l. Based on detailed analysis of pharmacokinetic data we developed three equations representing enzyme inhibition at bupivacaine concentrations of 0.1, 0.2 and 0.5 mmol/l. These equations allow us to calculate the acetylcholinesterase activity in solutions containing both cholinesterases utilizing the extinction differences measured spectrophotometrically in samples with and without bupivacaine. The accuracy of the bupivacaine-inhibition test could be confirmed by investigations on solutions of both purified cholinesterases and on samples of human cerebrospinal fluid. If butyrylcholinesterase activity has to be assessed simultaneously an independent test using butyrylthiocholine iodide as substrate (final concentration 5 mmol/l) has to be conducted.
Conclusions
The bupivacaine-inhibition test is a reliable method using spectrophotometrical techniques to measure acetylcholinesterase activity in cerebrospinal fluid. It avoids the use of toxic inhibitors for differentiation of acetylcholinesterase from butyrylcholinesterase in fluids containing both enzymes. Our investigations suggest that bupivacaine concentrations of 0.1, 0.2 or 0.5 mmol/l can be applied with the same effect using 1 mmol/l acetylthiocholine iodide as substrate.
Similar content being viewed by others
Background
AChE activity (E.C.3.1.1.7.) in CSF is assayed to study the function of cholinesterase releasing cells in the nervous system [1–8]. AChE and BChE (E.C.3.1.1.8.) are observed simultaneously in the CSF. Various methods to measure the enzymatic activity of AChE in CSF have been employed [9–16]. AChE hydrolyses only acetylcholine whereas BChE metabolizes acetylcholine as well as butyrylcholine. Overlapping substrate specificity thereby limits the use of colorimetric tests based on the assay reported by Ellman et al. [17]. Therefore the majority of colorimetric test procedures published to date are using relatively specific (highly toxic) inhibitors to distinguish the two different cholinesterases in the test system. We introduced an inhibitor-free test for AChE and BChE in the CSF [18]. During our recent studies we performed in vitro inhibition tests on AChE and BChE using various pharmacological agents [8]. We observed that a number of these pharmaceuticals can affect the test system by inhibiting the activity of cholinesterases. Interestingly pharmacokinetics of some drug-enzyme interactions were relatively specific suggesting bupivacaine to be a suitable agent for in vitro differentiation of cholinesterases.
The aim of this study was to develop an AChE assay for CSF using bupivacaine to inhibit BChE activity.
We performed in vitro tests using purified cholinesterases to define the bupivacaine concentration achieving maximum inhibition of BChE activity and at the same time influence AChE activity only to a minor degree. Based on pharmacokinetic analysis we developed equations, which allow us to calculate the AChE activity in solutions of both cholinesterases using the extinction differences measured spectrophotometrically in samples with and without bupivacaine. To confirm the correctness of these equations we applied the newly developed method to solutions of purified AChE and BChE as well as to samples of human lumbar CSF. The bupivacaine-inhibition test was also compared to the inhibitor-free test.
Results
AChE hydrolyses ACh with maximum activity at a final substrate concentration of 1 mmol/l. This substrate concentration was therefore used for all investigations. BChE shows only one quarter of its maximum activity at ACh final concentration of 1 mmol/l (Figure 1).
The influence of bupivacaine on the activity of both cholinesterases is demonstrated in Figure 2. AChE activity and BChE activity were determined in separate analyses. BChE inhibition above 95% of its activity was not achieved even at bupivacaine concentration of 1 mmol/l. The remaining BChE activity of 5% must therefore be added to all BChE activities measured under the influence of bupivacaine. Using drug concentrations which have minimum influence on AChE activity but cause maximum inhibition of BChE we selected three bupivacaine concentrations (0.1; 0.2 and 0.5 mmol/l) for further investigation (Figure 3).
99% of AChE- and 20% of BChE activity were detected (15% BChE activity measured plus 5% activity always remaining at higher bupivacaine concentration) following application of 0.1 mmol/l bupivacaine to the test system. Using 0.2 mmol/l bupivacaine 97% of AChE- and 15% of BChE activity persisted (10% BChE activity measured plus 5% activity always remaining). With 0.5 mmol/l bupivacaine 93% of AChE- and 10% of BChE activity persisted (5% BChE activity measured plus 5%).
Based on these findings the following equations for calculation of extinction differences (ΔEinhibited) at bupivacaine concentrations tested (0.1; 0.2 and 0.5 mmol/l) were created:
(1a) ΔEinhibited0.1 = 0.99 ΔEAChE + 0.20 ΔEBChE
(2a) ΔEinhibited0.2 = 0.97 ΔEAChE + 0.15 ΔEBChE
(3a) ΔEinhibited0.5 = 0.93 ΔEAChE + 0.10 ΔEBChE
Equation for the total extinction difference without bupivacaine inhibition (ΔEtotal):
(4a) ΔEtotal = 1.00 ΔEAChE + 1.00 ΔEBChE
Extracting ΔEAChE:
(4b) ΔEAChE = ΔEtotal - 1.00 ΔEBChE
(1b) ΔEAChE = 1.01 ΔEinhibited0.1 - 0.202 ΔEBChE
(2b) ΔEAChE = 1.03 ΔEinhibited0.2 - 0.155 ΔEBChE
(3b) ΔEAChE = 1.055 ΔEinhibited0.5 - 0.108 ΔEBChE
Introduction of equation (4b) into equations (1b) – (3b):
(1c) 0.798 ΔEBChE = ΔEtotal - 1.01 ΔEinhibited0.1
(2c) 0.845 ΔEBChE = ΔEtotal - 1.03 ΔEinhibited0.2
(3c) 0.892 ΔEBChE = ΔEtotal - 1.055 ΔEinhibited0.5
Extraction of ΔEBChE:
(1d) ΔEBChE = 1.253 ΔEtotal - 1.266 ΔEinhibited0.1
(2d) ΔEBChE = 1.183 ΔEtotal - 1.219 ΔEinhibited0.2
(3d) ΔEBChE = 1.120 ΔEtotal - 1.182 ΔEinhibited0.5
Introduction of equations (Id) – (3d) into equation (4b):
(1e) ΔEAChE = ΔEtotal - (1.253 ΔEtotal - 1.266 ΔEinhibited0.1)
(2e) ΔEAChE = ΔEtotal - (1.183 ΔEtotal - 1.219 ΔEinhibited0.2)
(3e) ΔEAChE = ΔEtotal - (1.120 ΔEtotal - 1.182 ΔEinhibited0.5)
Final equations to calculate ΔEAChE:
(1f) ΔEAChE = 1.266 ΔEinhibited0.1 - 0.253 ΔEtotal
(2f) ΔEAChE = 1.219 ΔEinhibited0.2 - 0.183 ΔEtotal
(3f) ΔEAChE = 1.182 ΔEinhibited0.5 - 0.120 ΔEtotal
As mentioned under methods ΔEAChE must be multiplied with factor 65 to calculate the AChE activity:
(1) AChE (nmol/min × ml) = 82.290 ΔEinhibited0.1 - 16.445 ΔEtotal
(2) AChE (nmol/min × ml) = 79.235 ΔEinhibited0.2 - 11.895 ΔEtotal
(3) AChE (nmol/min × ml) = 76.83 ΔEinhibited0.5 - 7.800 ΔEtotal
Purified enzymes
To prove the practical relevance of equations (1) – (3) we performed the following laboratory investigations. Samples of purified AChE and BChE were mixed in Ringer solution. We then measured the AChE activity using the bupivacaine-inhibition test and compared these results to the known enzyme activity in the solution. Using 0.1 mmol/l bupivacaine for inhibition of BChE we detected 99.2 ± 0.8% (94.7–103.3%) of the AChE activity originally set up in the mixture of both cholinesterases. The recovery rate for the AChE activity using 0.2 mmol/l bupivacaine was 99.7 ± 0.8% (94.0–105.0%). With 0.5 mmol/l bupivacaine we detected 98.2 ± 1.0% (94.7–100.4 %) of the original AChE activity. Detailed results are given in Table 1. The mean AChE activity detected with the bupivacaine-inhibition test (either bupivacaine concentration tested) did not differ significantly (Mann-Whitney-Test: p > 0.05) from the original enzyme activity in the solution with BChE.
We did not find significant differences between test results obtained with different bupivacaine concentrations (0.1; 0.2 and 0.5 mmol/l) comparing the mean recovery rates for AChE (Mann-Whitney-Test: p > 0.05).
CSF
Investigations were performed to check the suitability of the bupivacaine-inhibition test for lumbar CSF samples as source of cholinesterases. Comparison of mean recovery rates for AChE activity obtained using different bupivacaine concentrations (0.1; 0.2 and 0.5 mmol/l) did not show any significant differences between sample groups (Mann-Whitney-Test: p > 0.05).
We compared the AChE activity obtained with the bupivacaine-inhibition test to results acquired with the simultaneously conducted inhibitor-free test. AChE activity of CSF samples investigated varied from 8 to 35 nmol/ min × ml. Exact data can be obtained from Table 2.
On average the mean AChE activity measured with the inhibitor-free test was 3% lower than the activity measured using the bupivacaine-inhibition test (Wilcoxon-Test: p < 0.05). This difference was seen with all three bupivacaine concentrations used. To confirm this finding we calculated 103% of each individual test result obtained with the inhibitor-free method and compared these computed data to the bupivacaine-inhibition test results (Table 2). We could thereby eliminate significant differences between the mean AChE activity obtained by the two methods (Wilcoxon-Test: p > 0.05).
Discussion
The assay described in this paper is an elegant method to distinguish AChE- from BChE activity in body fluids containing both cholinesterases. This was achieved by using bupivacaine as a save alternative to the BChE inhibitor iso-OMPA [13, 16]. Bupivacaine proved to be a relatively selective BChE inhibitor in final concentrations from 1.0 to 5.0 × 10-4 mol/1, AChE inhibition remaining below 10%. Three equations (0.1; 0.2 and 0.5 mmol/l bupivacaine concentration) were generated after analysis of pharmacokinetic data (Figure 1).
The accuracy of equations (1) – (3) was first confirmed by a test series detecting the AChE activity in solutions of purified AChE and BChE. Alltogether 99.3 ± 0.5 % of the AChE activity originally set up in test solutions were detected. There were no significant differences comparing the mean AChE recovery rates obtained with 0.1 mmol/l; 0.2 mmol/l or 0.5 mmol/l bupivacaine.
Furthermore the relevance of the bupivacaine-inhibition test was confirmed by analysis of human CSF samples. Comparison of the mean AChE recovery rates did not reveal significant differences between test results acquired with either 0.1 mmol/l; 0.2 mmol/l or 0.5 mmol/l bupivacaine. All three drug concentrations can therefore be applied equally with the bupivacaine-inhibition test.
Until recently we have been using the inhibitor-free test, which permits for simultaneous analysis of both cholinesterases in the CSF [18]. The inhibitor-free method is based on the finding that the ratio of individual activities of BChE on its both substrates ACh and BCh in the CSF and in the serum obtained at the same time are identical with substrate concentrations of at least 5 mmol/l. It became obvious that at 5 mmol/l ACh concentration the AChE activity already shows moderate inhibition by its substrate (Figure 1). This substrate inhibition evidently causes about 3% lower enzyme activity measured with the inhibitor-free test in comparison to the bupivacaine-inhibition test (Table 2). However, the AChE activity was not inhibited by ACh at 1 mmol/l substrate concentration (Figure 1) which is used for the bupivacaine-inhibition test. We were able to show that significant mean AChE activity differences between the two test procedures could be eliminated adding 3% of the enzyme activity measured with the inhibitor-free procedure to the original data obtained by this method (Table 2).
It does not appear advisable to judge whether the bupivacaine-inhibition test or the inhibitor-free test is more precise when one considers the minor differences between test results. We could previously show that the inhibitor-free procedure offers widely identical results in comparison to the difference method using BW284c51 as AChE-inhibitor which proved to be a trustworthy assay [9, 11, 12, 15, 18]. We could also show that the assay utilizing iso-OMPA as inhibitor for BChE exclusively [14] is unreliable if applied without correction of methodical errors [18]. So far no substance has been found which completely inhibits BChE without also affecting AChE. Bupivacaine is no exception. It is thus obvious that any procedure computing the AChE activity after inhibition of either cholinesterase has its disadvantages. However incomplete inhibition of BChE and additional AChE inhibition can be handled by studying pharmacokinetics (known enzyme activity and inhibitor concentration) to precisely determine the impact of these unwanted effects.
The inhibitor-free test and the bupivacaine-inhibition test are both accurate assays to measure the AChE activity in CSF. The inhibitor-free procedure requires an additional serum sample to be taken at the same time as the CSF sample and is therefore a little more expensive than the bupivacaine-inhibition test. Still the inhibitor-free test should be the method of choice if both cholinesterases in the CSF and the BChE activity in the serum have to be assessed simultaneously. If only the AChE activity has to be assayed the bupivacaine-inhibition test ought to be used because it is cheaper and easy to perform. Bupivacaine is a save inhibitor and could even be provided together with the substrate ACh, DTNB and eserine salizylate in a standardized test kit.
BChE activity in serum or CSF can of course be tested independently employing the substrate butyrylthiocholine iodide at a final concentration of 5 mmol/l. BChE shows its full activity only at this substrate concentration (Figure 1), AChE being unable to compromise the test result because of its substrate specificity.
Conclusions
The bupivacaine-inhibition test is a reliable method to measure AChE activity in CSF. It avoids the use of toxic inhibitors to differentiate AChE- from BChE activity in fluids containing both enzymes. Our investigations suggest that bupivacaine concentrations of 0.1, 0.2 or 0.5 mmol/l can be applied equally using the substrate acetylthiocholine iodide at a final test concentration of 1 mmol/l.
Material and Methods
Purified AChE (cholinesterase, acetyl C 2888 Type V-S: from electric eel; 1000–2000 U/mg) and purified BChE (cholinesterase, butyryl C 4290 from horse serum, highly purified minimum 500 U/mg) were obtained from Sigma-Aldrich Co. Acetylthiocholine iodide >/= 99% (AT), butyrylthiocholine iodide ~99% (AT), 5-5'dithio bis-(2-nitrobenzoic acid) (3,3'-6) ~99% (HPLC) and eserine salizylate were obtained from Fluka BioChemika. Bupivacaine-hydrochloride (Carbostesin® 0.25%) was obtained from Astra GmbH Wedel, Germany.
Enzyme assay
AChE- and BChE activity were measured spectrophotometrically applying the technique of Ellman et al. [17]. Samples containing purified AChE and BChE dissolved in Ringer (0.1 ml solution) and samples of human lumbar CSF (0.1 ml; cell-free) were investigated. The substrate ACh was used at a final concentration of 1 mmol/l. The final concentration of DTNB was 1 mmol/l. The reaction was performed at 37°C in a total volume of 1.3 ml and stopped after 20 min linear reaction time using 0.5 ml of 0.1 mmol/l eserine salizylate. The reaction product 5-thio-2-nitrobenzoate produced enzymatically was measured at 412 nm. AChE activity was calculated by multiplication of the extinction difference (ΔEAChE) with factor 65 which results from the volume of the reactants, the total volume of 1.8 ml the reaction time of 20 min and the millimolar extinction coefficient of 13.88.
The inhibitor-free test was described in detail in one of our previous publications [18].
Tests using purified enzymes
Test-series were performed on samples containing purified AChE and BChE. The inhibition effect of three different bupivacaine concentrations (0.1; 0.2 and 0.3 mmol/l) was investigated:
60 samples (mean AChE activity: 43.5 ± 1.3 nmol/ min × ml; 35.8 – 56.5 nmol/ min × ml) were tested under the influence of 0.1 mmol/l bupivacaine.
85 samples (mean AChE activity: 49.8 ± 2.7 nmol/ min × ml; 25.6 – 64.5 nmol/ min × ml) were investigated using 0.2 mmol/l bupivacaine.
24 samples (mean AChE activity: 51.2 ± 6.2 nmol/ min × ml; 29.7 – 71.2 nmol/ min × ml) and BChE were tested with 0.5 mmol/l bupivacaine.
Tests using human CSF
Investigations were performed to check the applicability of the bupivacaine-inhibition test for human lumbar CSF. Samples remaining after completed routine laboratory analysis were used. We compared the mean recovery rates for AChE activity obtained under the influence of different bupivacaine concentrations (0.1; 0.2 and 0.5 mmol/l). Lumbar CSF samples were also used to compare the bupivacaine-inhibition test with the inhibitor-free test. Three test-series were performed. 45 CSF samples were examined applying 0.1 mmol/l bupivacaine and 0.2 mmol/l bupivacaine respectively. 8 CSF samples were assayed under the influence of 0.5 mmol/l bupivacaine.
Statistical tests
Data are given as means ± SEM. We used nonparametric tests to compare means of sample groups (Mann-Whitney-Test for independent sample groups and Wilcoxon-Test for dependent sample groups). The level of significance was set at 0.05. Tests were performed using SPSS 9.0 for Windows®.
Abbreviations
- BW284c51:
-
1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-one dibromide
- ΔE:
-
extinction difference
- ΔEinhibited:
-
extinction difference measured under the influence of bupivacaine
- ΔEtotal:
-
extinction difference measured without bupivacaine
- ACh:
-
acetylthiocholine iodide
- AChE:
-
acetylcholinesterase
- BCh:
-
butyrylthiocholine iodide
- BChE:
-
butyrylcholinesterase
- CSF:
-
cerebrospinal fluid
- DTNB:
-
5-5'dithio bis-(2-nitrobenzoic acid) (3,3 '-6)
- iso-OMPA:
-
tetraisopropylpyrophosphoramide
References
Dickmann UK, Soerensen K, Wiedemann T, Mader M, Felgenhauer K: Neuronal acetylcholinesterase in serum and CSF: A prototypic marker for the brain-blood-transfer of proteins?. J clin Chem clin Biochem. 1989, 27: 836-
Koponen HJ, Riekkinen PJ: Cerebrospinal fluid acetylcholinesterase in patients with dementia associated with schizophrenia or chronic alcoholism. Acta Psychiatr Scand. 1991, 83: 441-443.
Hartikainen P, Soininen H, Partanen J, Helkala EL, Riekkinen P: Aging and spectral analysis of EEG in normal subjects: a link to memory and CSF AChE. Acta Neurol Scand. 1992, 86: 148-155.
Basu PS, Batabyal SK, Bhattacharya A, Datta TK: Cholinesterase activities in cerebrospinal fluid of patients with idiopathic convulsive disorders. Clin Chim Acta. 1995, 235: 107-112. 10.1016/0009-8981(95)06016-5.
Konings CH, Kuiper MA, Mulder C, Calliauw J, Wolters EC: CSF acetylcholinesterase in Parkinson disease: decreased enzyme activity and immunoreactivity in demented patients. Clin Chim Acta. 1995, 235: 101-105. 10.1016/0009-8981(95)06004-9.
McQueen MJ: Clinical and analytical considerations in the utilization of cholinesterase measurements. Clin Chim Acta. 1995, 237: 91-105. 10.1016/0009-8981(95)06067-N.
Kluge WH, Kluge HH, Hochstetter A, Vollandt R, Bauer HI, Venbrocks R: Butyrylcholinesterase in Lumbar and Ventricular Cerebrospinal Fluid. Acta Neurol Scand. 2001, 104: 17-23. 10.1034/j.1600-0404.2001.00286.x.
Kluge WH, Kluge HH, Hochstetter A, Vollandt R, Seidel F, Venbrocks R: Acetylcholinesterase in lumbar and ventricular cerebrospinal fluid. Clinica Chimica Acta. 2001, 305: 55-63. 10.1016/S0009-8981(00)00423-X.
Arendt T, Bigl V, Walther F, Sonntag M: Decreased ratio of CSF acetylcholinesterase to butyrylcholinesterase activity in Alzheimer's disease. Lancet. 1984, 21: 173-10.1016/S0140-6736(84)90116-8.
Huff FJ, Maire JC, Growdon JH, Corcin S, Wurtmann RC: Cholinesterases in cerebrospinal fluid-correlations with clinical measures in Alzheimer's disease. J Neurol Sci. 1986, 72: 121-129. 10.1016/0022-510X(86)90001-8.
Atack JR, May C, Kaye JA, Kay AD, Rapoport SI: Cerebrospinal fluid Cholinesterases in aging and in dementia of the Alzheimer type. Ann Neurol. 1988, 23: 161-167.
Brimijoin S, Hammond P: Butyrylcholinesterase in human brain and acetylcholinesterase in human plasma: Trace enzymes measured by two-site immunoassay. J Neurochem. 1988, 51: 1227-1231.
Manyam BV, Giacibini E, Colliver JA: Cerebrospinal fluid acetylcholinesterase and choline measurements in Huntington's disease. J Neurol. 1990, 237: 281-284.
Kumar V, Giacobini E, Markwell S: CSF choline and acetylcholinesterase in early-onset vs. late-onset Alzheimer's disease patients. Acta Neurol Scand. 1989, 80: 461-466.
Appleyard ME, McDonald B: Acetylcholinesterase and butyrylcholinesterase activities in cerebrospinal fluid from different levels of the neuraxis of patients with dementia of the Alzheimers type. J of Neurology, Neurosurgery and Psychiatry. 1992, 55: 1074-1078.
Tornel PL, Campoy FJ, Vidal CJ: Cholinesterases in cerebrospinal fluid in patients with meningitis and hydrocephaly. Clin Chim Acta. 1993, 214: 219-225. 10.1016/0009-8981(93)90113-I.
Ellman GL, Courtney KD, Andrews Vjr, Featherstone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961, 7: 88-95. 10.1016/0006-2952(61)90145-9.
Kluge HH, Kluge WH, Hartmann W: An inhibitor-free assay of acetylcholinesterase and butyrylcholinesterase in the cerebrospinal fluid. Clinica Chimica Acta. 1999, 282: 135-145. 10.1016/S0009-8981(99)00021-2.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kluge, W.H., Kluge, H.H., Bauer, H.I. et al. Acetylcholinesterase assay for cerebrospinal fluid using bupivacaine to inhibit butyrylcholinesterase. BMC Biochem 2, 17 (2001). https://doi.org/10.1186/1471-2091-2-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2091-2-17