Abstract
Background
Glioblastoma multiform (GBM) is a devastating brain tumor with maximum surgical resection, radiotherapy plus concomitant and adjuvant temozolomide (TMZ) as the standard treatment. Diverse clinicopathological and molecular features are major obstacles to accurate predict survival and evaluate the efficacy of chemotherapy or radiotherapy. Reliable prognostic biomarkers are urgently needed for postoperative GBM patients.
Methods
The protein coding genes (PCGs) and long non-coding RNA (lncRNA) gene expression profiles of 233 GBM postoperative patients were obtained from The Cancer Genome Atlas (TCGA), TANRIC and Gene Expression Omnibus (GEO) database. We randomly divided the TCGA set into a training (n = 76) and a test set (n = 77) and used GSE7696 (n = 80) as an independent validation set. Survival analysis and the random survival forest algorithm were performed to screen survival associated signature.
Results
Six PCGs (EIF2AK3, EPRS, GALE, GUCY2C, MTHFD2, RNF212) and five lncRNAs (CTD-2140B24.6, LINC02015, AC068888.1, CERNA1, LINC00618) were screened out by a risk score model and formed a PCG-lncRNA signature for its predictive power was strongest (AUC = 0.78 in the training dataset). The PCG-lncRNA signature could divide patients into high- risk or low-risk group with significantly different survival (median 7.47 vs. 18.27 months, log-rank test P < 0.001) in the training dataset. Similar result was observed in the test dataset (median 11.40 vs. 16.80 months, log-rank test P = 0.001) and the independent set (median 8.93 vs. 16.22 months, log-rank test P = 0.007). Multivariable Cox regression analysis verified that it was an independent prognostic factor for the postsurgical patients with GBM. Compared with IDH mutation status, O-(6)-methylguanine DNA methyltransferase promoter methylation status and age, the signature was proved to have a superior predictive power. And stratified analysis found that the signature could further separated postoperative GBM patients who received TMZ-chemoradiation into high- and low-risk groups in TCGA and GEO dataset.
Conclusions
The PCG-lncRNA signature was a novel prognostic marker to predict survival and TMZ-chemoradiation response in GBM patients after surgery.
Similar content being viewed by others
Background
Glioblastoma multiforme (GBM) is regarded as the most common malignant brain tumor in adults, accounting for 47.1% of all malignant brain tumors [1], and the median survival time of untreated patients with GBM is only 3 months [2]. For malignant brain tumors, according to the Central Brain Tumor Registry of the United States (CBTRUS), the incidence rate of GBM in the United States is extremely high (3.20/100,000 population) and increases with age [1]. Maximal surgical resection, is considered as the first-line treatment for GBM patients relieving clinical symptoms, extending survival time and providing tissue to pathological diagnosis [3]. A large-scale randomized phase III trial, initiated by the European Organization for Research and Treatment of Cancer and National Cancer Institute of Canada Clinical Trials Group, found that the 2-year survival rate of GBM patients was improved to 26.5% by radiotherapy plus temozolomide from 10.4% by radiotherapy alone [4]. Since then, the standard therapeutic strategy for glioblastoma patients has become the multimodal treatment with radiotherapy and chemotherapy after surgery. Therefore, prediction of response to chemotherapy drugs or radiation and prediction of prognosis are crucial for post-surgical GBM patients.
In 1993, the Radiation Therapy Oncology Group-Recursive Partitioning Analysis (RTOG-RPA) classification system was developed for high-grade glioma patients with similar survival times [5] and validated its prognostic significance in GBM patients [6,7,8]. However, all the stratification variables of RTOG-RPA risk classification are clinical factors including age, tumor size and location, treatment, karnofsky performance score (KPS), cytologic, histologic composition and so on. Due to the intra- and inter-individual heterogeneity, the RTOG-RPA classification could not satisfactorily predict the survival and tumor response to therapy of each individual [9]. Therefore, molecular markers are becoming more useful in the field of prognosis prediction [10]. Currently, GBM related researches from genomics, epigenomics and transcriptomics level have led to unprecedented discoveries of potential prognostic and predictive indicators [11]. Genomic analysis suggests survival-related genomic abnormalities in GBM patients, such as epidermal growth factor receptor (EGFR) amplification [12, 13] and isocitrate dehydrogenase 1/2 (IDH1/2) mutations [14, 15], have prognostic value. Some studies show that high expression of EGFR indicated poor prognosis [16], and other research find the IDH mutations are associated with improved survival [17]. From the epigenetic level, O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation has been demonstrated that it is associated with improved progression-free and overall survival in GBM patients treated with alkylating agents [18]. However, genomic prognostic classification of GBM is not yet clinically feasible, and the mechanism of how these multiple genomic alterations affect clinical prognosis is not clear [19].
As far as the transcriptomics level is concerned, studies mostly focus on mRNA or protein coding gene (PCG) and long noncoding gene (lncRNA) because of their role as gene expression regulators, tumor suppressors and oncogenes. Using PCGs or lncRNAs, numerous studies have constructed transcriptome prognosis models for GBM survival prediction. Zhu et al. screened out an effective prediction system composed of 63 signature genes for glioblastoma prognosis [20]. Marko et al. identified a 43-gene “fingerprint” from a population of 1478 differential expressed genes (P < 0.01) that distinguished GBM survival phenotypes [21]. Anindya Dutta et al. in a global analysis identified 584 lncRNAs correlated with a poor prognosis and 282 lncRNAs associated with better survival outcomes in GBM patients [22]. Above researches verify PCGs and lncRNAs can be prognostic biomarkers of GBM. However, these studies found too many prognostic genes to provide a clinically feasible transcriptome signature with a small number of genes to predict the survival of GBM patients. Therefore, we focus our attention on find out a molecular signature which contains few prognostic genes and could more accurately predict the outcomes of postoperative GBM patients and guide the tailored therapy.
In the present study, we sought to explore the role of multi-transcriptome signature in the prognosis of GBM patients after surgery. We analyzed 233 postoperative GBM patients with the expression profiles of mRNAs and lncRNAs and screened out genes significantly associated with survival. Through further bioinformatics analysis, we aimed at constructing a prognostic transcriptome signature to divide patients into different risk groups, thereby assessing the survival and treatment response for GBM patients after surgical resection.
Methods
Glioblastoma multiforme datasets
We downloaded the normalized TCGA level 3 mRNA expression data and corresponding clinical information of GBM patients (n = 153) from the UCSC Xena (https://gdc.xenahubs.net/download/TCGA-GBM/Xena_Matrices/TCGA-GBM.htseq_fpkm.tsv.gz). LncRNA expression data of the corresponding GBM patients was obtained from the TANRIC database (https://ibl.mdanderson.org/tanric/_design/basic/download.html) [23]. Another part GBM expression data (GSE7696, n = 80) and corresponding clinical data was obtained from the publicly available GEO database (https://www.ncbi.nlm.nih.gov/geo/). GSE7696 data was generated by the Affymetrix Human Genome U133 Plus 2.0 Array (http://www.affymetrix.com/support/technical/byproduct.affx?product=hg-u133-plus) and included 80 tumors and 4 normal samples. By probe re-annotation [24], we got their PCG and lncRNA expression data. Then we processed the gene expression data by removing the genes with missing expression values in more than 30% of samples or patients and excluding genes whose expression value were 0 or null [25]. For the remaining genes with missing expression value in less than 30% of samples or patients, we used the mean of the corresponding genes expression values by R program to replace the missing expression values. We used the expression value on a log2 scale in the subsequent analysis.
A total of 233 glioblastoma patients concurrent with gene expression profiles and clinical information were utilized in our study. Of these, all GBM patients were postoperative, then treated with radiotherapy or chemotherapy. The 153 GBM patients from TCGA database were randomly assigned to a training set (n = 76) or a testing set (n = 77) using the ‘sample’ function [26] from R library and the 80 patients from GSE7696 were served as an independent validation set. Table 1 described the clinical characteristics and therapy information of the TCGA and GEO cohort respectively.
Construction of the prognostic PCG-lncRNA signature in the training dataset
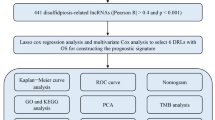
The relationship between the expression of PCG or lncRNA and patients’ overall survival (OS) was analyzed by univariate Cox proportional hazards regression analysis in the training dataset. Genes were selected if P value < 0.05. Before constructing a risk prediction model, the random survival forests-variable hunting (RSFVH) algorithm was performed to filter genes. In the random survival forest supervised classification algorithm, an iteration procedure was implemented to narrow down the gene set, and each iteration step discarded the 1/4 least important PCGs or lncRNAs. One thousand trees were grown at each step, and the square root of the number of input nodes at each step was set to the size of randomly chosen PCGs or lncRNAs at each node of single classification tree. Because the number of good-prognostic and poor-prognostic patients were not equal, the class weights were adjusted accordingly. The generalization error was estimated on the out-of-bag samples. Finally, six PCGs and six lncRNAs were selected [27,28,29]. Risk prediction score model was developed by these selected genes, weighted by their estimated regression coefficients as follows [30].
where N is the number of prognostic lncRNAs or PCGs, Expi is the expression value of lncRNAs or PCGs, and Coei is the estimated regression coefficient of PCGs or lncRNAs in the univariate Cox regression analysis. Then each patient obtained 4095 risk scores because six PCGs and six lncRNAs could form 212 − 1 = 4095 combinations or signatures. The receiver operating characteristic (ROC) curve was used to compare the sensitivity and specificity of the 4095 signatures in the training dataset. Area under the curve (AUC) were calculated from the ROC curve. By comparing the AUC values, we selected the prognostic PCG-lncRNA signature in the training set.
Statistical analysis and bioinformatics analysis
With the median risk score in the training dataset as the cutoff value, the GBM patients in training or test set were divided into high-risk or low-risk group [31]. In GSE7696, X-tile software was used to select cutoff value for risk grouping [32]. The Kaplan–Meier analysis and the log-rank test were used to assess and compare survival differences between the low-risk and high-risk groups. ROC analysis was tested to compare the survival predictive power. Furthermore, to test whether the signature was an independent prognostic factor, multivariable Cox regression analysis and data stratification analysis were performed. All analyses were performed using R program 3.2.3 (http://www.r-project.org) including packages named pROC, survival and randomForestSRC downloaded from Bioconductor.
To investigate the biological roles of the PCGs and lncRNAs in the signature, we analyzed the co-expressed protein coding genes of the prognostic genes computed by Pearson correlation test and genes with P < 0.05 and absolute value of the Pearson coefficient > 0.4 were selected. Here, SubpathwayMiner was used for identification of related pathways of the selected genes (http://cran.r-project.org/web/packages/SubpathwayMiner/) for it supports multiple species (approximately 100 eukaryotes, 714 bacteria and 52 Archaea) and different gene identifiers (Entrez Gene IDs, NCBI-gi IDs, UniProt IDs, PDB IDs, etc.) in the KEGG GENE database, which provides more flexibility in annotating gene sets and identifying the involved pathways (entire pathways and sub-pathways) [33].
Results
Characteristics of study subjects
In this study, the GBM patients after surgical resection and their expression profiles were used as the main subjects. After screened the data downloaded from the TCGA, TANRIC and GEO database, we identified 233 eligible patients diagnosed with GBM concurrently including PCG and lncRNA expression profiles and corresponding clinical data. All these GBM patients received surgical treatment and the median age of the enrolled patients was 60 years (21–89 years). Simultaneously, we obtained a total of 14,607 PCGs and 6613 lncRNAs from the 233 GBM patients.
Identification of the prognostic PCG-lncRNA signature in the training dataset
Firstly, in order to find the survival-related genes in training set, univariate cox proportional hazards regression analysis was performed and identified a 707-genes set including 437 PCGs and 270 lncRNAs in the training dataset which were significantly correlated with OS (P < 0.05, Additional file 1: Table S1). The volcano plot displayed the 707 genes with statistical differences as the blue dots in Fig. 1a. Secondly, to further narrow down the number of prognostic PCGs or lncRNAs, we analyzed the above 707 survival related genes by random survival forest algorithm and got six PCGs (EIF2AK3, EPRS, GALE, GUCY2C, MTHFD2, RNF212) and six lncRNAs (LINC00618, LINC02015, AC068888.1, CERNA1, CTD-2140B24.6, ZMIZ1-AS1) significantly associated to OS of GBM patients according to the permutation important score in every step: Discard 1/4 less important PCGs and lncRNAs at each step based on estimating the important score for each PCG or lncRNA using the out-bag samples by pemutation testing (Fig. 1b–d).
Identification of the prognostic PCG-lncRNA signature in the training dataset. a Volcano plot of the survival associated lncRNAs and PCGs in univariate cox regression analysis. b The random survival forest analysis of the survival related 707-gene set in postsurgical GBM patients. c, d The important score of selected PCGs or lncRNAs which were calculated by random survival forest analysis. e The 11 genes with largest AUC (k = 1, 2, … 12, k represents the gene number in the corresponding signature) were shown in the plot. f After calculating the AUC of 4095 signatures, the prognostic PCG-lncRNA signature with biggest predictive power (AUC = 0.78) was screen out
Thirdly, putting the six lncRNAs and six PCGs into the risk score model constructed in methods, we obtained a total of 212 − 1 = 4095 models or signatures that included different gene numbers from 1 to 12, indicating that PCGs and lncRNAs alone or the combination of PCGs and lncRNAs were included in these 4095 models. To screen out a signature with biggest predictive power, we performed ROC analysis 4095 times using the survival status as label and signature risk scores of GBM patients as variable in the training dataset by pROC R packages. After compared the AUC values of all these 4095 signatures (Additional file 2: Table S2), we identified the max AUC value was 0.78 (Fig. 1e, f) from the PCG-lncRNA signature comprising six PCGs (EIF2AK3, EPRS, GALE, GUCY2C, MTHFD2, RNF212) and five lncRNAs (LINC00618, LINC02015, AC068888.1, CERNA1 and CTD-2140B24.6).
The risk score model was constructed as follows: Risk score = (− 0.82 × EIF2AK3 expression) + (− 0.79 × EPRS expression) + (0.71 × GALE expression) + (0.60 × GUCY2C expression) + (− 0.71 × MTHFD2 expression) + (− 0.87 × RNF212 expression) + (0.57 × LINC00618 expression) + (− 0.84 × LINC02015 expression) + (0.61 × AC068888.1 expression) + (0.65 × CERNA1 expression) + (− 0.74 × CTD-2140B24.6 expression). Among them, the coefficients for PCGs (EIF2AK3, EPRS, MTHFD2 and RNF212) and lncRNAs (LINC02015, CTD-2140B24.6) are negative, and the coefficients for PCGs (GALE, and GUCY2C) and lncRNAs (LINC00618, AC068888.1 and CERNA1) are positive (Table 2).
Validation the survival prediction of the PCG-lncRNA signature in the three dataset
The risk score model constructed by the PCG-lncRNA signature in the training dataset gave each patient a risk score. Patients from the training dataset were divided into high-risk group (n = 38) and low-risk group (n = 38) when the median risk score was used as the cutoff point. Kaplan–Meier survival analysis was performed to compare the overall survival of two risk groups of patients. As we can see in Fig. 2a, the OS rates were significantly different in patients from the two groups. Compared with those in the low-risk group, patients in the high-risk group had a shorter survival time (median survival: 7.47 months vs. 18.27 months, log-rank test P < 0.001) and lower OS rate (5% vs. 50%, log-rank test P < 0.001, Fig. 2a).
The PCG-lncRNA signature predicts survival of postoperative GBM patients in the training, test and independent validation set. Kaplan–Meier survival curves classify training-set patients (n = 76) (a) test-set patients (n = 77) (b) and independent validation set (n = 80) (c) into high- and low-risk groups by the PCG-lncRNA signature. Shorter survival time was noted in GBM patients with higher risk-scores in the training (d), test (e) and independent validation (f) datasets
To validate the predictive power of the signature, we calculated the PCG-lncRNA signature-based risk scores of 77 patients in the test dataset. When the same median cutoff point obtained from the training dataset was used, patients from the test dataset were also separated into low-risk and high-risk groups (median survival: 11.4 months vs. 16.8 months, log-rank test P = 0.001, Fig. 2b). The OS rate of patients in the high-risk group was about 19.4% vs. 53.7% in the low-risk group (Fig. 2b). In the independent set, Kaplan–Meier analysis found the PCG-lncRNA signature classified patients into different two risk groups (median survival: 8.93 months vs. 16.22 months, log-rank test P = 0.007, Fig. 2c). Moreover, shorter survival time was noted in GBM patients with higher risk-scores in the training, test and independent datasets and P-values were calculated by the rank-sum test (Fig. 2d–f).
The PCG-lncRNA signature is an independent prognostic factor from other clinical variables and molecular features
After demonstrating the survival predictive power of PCG-lncRNA signature, we need to clarify whether the PCG-lncRNA signature was an independent prognostic factor since numerous factors affect GBM prognosis. Thus we performed univariable cox analysis and multivariable Cox regression analysis in which covariates included the PCG-lncRNA signature-based risk score and clinical features (Table 3). Multivariable Cox regression analysis showed that the PCG-lncRNA risk score remained to be significantly associated with overall survival when adjusted other clinical features including sex, age and Karnofsky performance score in the training and the test dataset (High-risk group vs. Low-risk group, HR = 5.94, 95% CI 2.66–13.25, P < 0.001; HR = 2.89, 95% CI 1.35–6.20, P = 0.01). The independent dataset showed the independent predictive power of PCG-lncRNA signature (HR = 2.17, 95% CI 1.16–4.07, P = 0.02) when adjusted other clinical features including sex, age.
Subsequently, examining the clinical data of these 153 TCGA GBM patients after surgical resection, we obtained 73 samples with known status of MGMT promoter and 80 samples with known status of IDH1 mutation (both known were 70 samples). Multivariable Cox regression analysis showed that the PCG-lncRNA risk score was significantly associated with overall survival when adjusted the molecular features including MGMT promoter and IDH1 mutation in the 70 TCGA GBM patients (High-risk group vs. Low-risk group, HR = 3.71, 95% CI 1.80–7.62, P < 0.001).
Comparing the survival predictive power of the signature with that of age, IDH1 mutation and MGMT promoter methylation status
To compare the survival predictive power of the PCG-lncRNA signature with the reported prognostic factors, such as age, IDH1 mutation and MGMT promoter methylation status, we performed a series of ROC analyses considering that a larger AUC usually represented a better predictive power [34, 35].
In the training dataset (n = 76), the AUC of the PCG-lncRNA signature was bigger than that of age, indicating a better predictive power in GBM prognosis (Signature-AUC = 0.78 vs. Age-AUC = 0.56, Fig. 3a). The same result can be seen in the test dataset (Signature-AUC = 0.69 vs. Age-AUC = 0.53, n = 77, Fig. 3b), Furthermore, the AUC of the signature model combined with age was maximum (Age + Signature-AUC = 0.80/0.68 in training/test group, Fig. 3a, b), illustrating combination of the PCG-lncRNA signature with age could provide more precisely prognostic information. And we also compared the survival predictive ability at 1, 2 and 3 years of the PCG-lncRNA signature with that of age by TimeROC analysis in the entire TCGA 153 samples. As Fig. 3c showed, the AUC of the PCG-lncRNA signature is 0.69 (0.60–0.77) at 1 year, 0.72 (0.60–0.83) at 2 year and 0.81 (0.76–0.86) at 3 year, larger than that of age 0.60 (0.51–0.69) at 1 year, 0.60 (0.48–0.73) at 2 year and 0.57 (0.37–0.76) at 3 year.
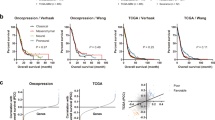
ROC analyses for comparison of the predictive power of the PCG-lncRNA signature with that of age in the training (a), test dataset (b) and TimeROC analysis in the entire set (c). TimeROC analysis for comparison of the survival predictive power of the PCG-lncRNA signature with that of MGMT promoter methylation status (d) and IDH1 mutation (g). Kaplan–Meier survival curves found the PCG-lncRNA signature (e, h) could classify patients with known MGMT promoter (f) and IDH1 mutation status (i) into high- and low-risk groups by the PCG-lncRNA signature in each corresponding TCGA dataset. Kaplan–Meier survival curves found the MGMT and IDH1/2 could not group patients into high- and low-risk groups
Then, we compared the survival predictive power of the PCG-lncRNA signature with MGMT or IDH1 mutation by TimeROC analysis. For MGMT set (n = 73), the AUC of the signature was 0.78 (0.67–0.90) at 1 year and 0.79 (0.70–0.88) at 2 year, larger than that of MGMT which was 0.53 (0.38–0.65) at 1 year and 0.53 (0.26–0.78) at 2 year, but the AUC of the signature was 0.77 (0.69–0.85) at 3 year a little less than that of MGMT which was 0.78 (0.69–0.86) (Fig. 3d). For IDH1 mutation set (n = 80), the AUC of the PCG-lncRNA signature was 0.74 (0.62–0.86) at 1 year, 0.79 (0.61–0.88) at 2 year and 0.76 (0.68–0.84) at 3 year, larger than that of IDH1 mutation which was 0.47 (0.43–0.51) at 1 year, 0.40 (0.22–0.58) at 2 year and 0.52 (0.48–0.57) at 3 year (Fig. 3g).
In addition, Kaplan–Meier survival analysis was performed in the 73 samples with known status of MGMT promoter and 80 samples with known status of IDH1 mutation to compare the risk grouping ability of the PCG-lncRNA signature with that of MGMT and IDH1 mutation. Using the same median cutoff point obtained from the training dataset, the PCG-lncRNA signature showed a robust efficiency to separate corresponding patients into two risk groups with different survival time (P < 0.001, Fig. 3e, h), however, the MGMT and IDH1/2 did not group well (P > 0.05, Fig. 3f, i).
Stratification analysis of TMZ-chemoradiation treatment
The relationship between the PCG-lncRNA signature with a series of clinicopathological parameters in the entire TCGA dataset (n = 153) was analyzed. As can be seen in Table 4, there was an association between PCG-lncRNA signature and TMZ-chemoradiation (Chi square test, P < 0.05, Table 4). Obviously, TMZ-chemoradiation treatment could stratify post-operative GBM patients into treated stratum and untreated stratum. Data stratification analysis using the PCG-lncRNA signature risk score further divided the patients into four groups: high-risk and treated, high-risk and untreated, low-risk and treated, low-risk and untreated. The Kaplan–Meier test was performed and Kaplan–Meier curves showed in Fig. 4. The log-rank test showed that TMZ-chemoradiation treated patients in high-risk group with shorter survival than TMZ-chemoradiation treated patients in low-risk group (n = 62, P < 0.001, Fig. 4a). The TMZ-chemoradiation untreated patients were also divided into a high-risk group with lower OS and a low-risk group with higher OS (n = 83, P = 0.005, Fig. 4b), indicating the stratification power of the PCG-lncRNA signature in TMZ-chemoradiation GBM patients.
Stratification analysis in the entire set. Kaplan–Meier survival curves found the PCG-lncRNA signature could classify TMZ-chemoradiation treated patients (a) and untreated patients (b) in the entire TCGA dataset and TMZ-chemoradiation treated patients (c) and untreated patients (d) in the GSE7696 dataset into high- and low-risk groups. P Value was calculated by log-rank test
In consistence with the findings in TCGA described above, for GSE7686 dataset, the PCG-lncRNA signature could stratify the TMZ-chemoradiation treated or untreated patients into a high-risk group and a low-risk group with different survival (log-rank test P = 0.03, Fig. 4c, log-rank test P = 0.13, Fig. 4d).
Functional characterization of the prognostic genes in the PCG-lncRNA signature
Co-expression network analysis was carried out in the entire TCGA dataset visualized by Cytoscape [36] and we found 2328 protein-coding genes co-expressed with the prognostic 6 PCGs and 5 lncRNAs in the signature (Absolute value of the Pearson correlation coefficient > 0.40, P < 0.05, Additional file 3: Table S3). Then we performed pathway analysis by SubpathwayMiner (see method) and found these co-expressed genes were enriched in 90 different pathways (P < 0.05, Additional file 4: Table S4). The gene set were significantly associated with different cancer types such as non-small cell lung cancer, prostate cancer, thyroid cancer, bladder cancer and glioma (P < 0.05, Fig. 5a). And these results suggested that the 11 genes, via the co-expressed genes, could exert their regulatory roles by implicating in regulating downstream pathways such as JAK-STAT signaling pathway, MAPK signaling pathway, WNT signaling pathway, Cell cycle, TGF-beta signaling pathway and p53 signaling pathway (P < 0.05, Fig. 5b, c).
Discussion
Glioblastoma multiforme (GBM) is a heterogeneous disease characterized by poor prognosis. In order to extend the survival time of patients with GBM, in recent year, adjuvant and concomitant temozolomide with radiation are widely used. Despite advances in treatment such as radiation and chemotherapy, the prognosis and therapy response for post-surgical GBM patients with similar clinical risk factors varied tremendously. Considering the molecular heterogeneity of GBM, in this study, we identified a prognostic molecular indicator comprising five long non-coding RNAs and six protein coding genes, and confirmed the survival prediction power of the PCG-lncRNA signature in postoperative GBM patients.
Molecular markers are of great significance to disease diagnosis, treatment decision and prognosis assessment. With regard to the prognostic molecular characterization of GBM, the 2016 World Health Organization (WHO), for the first time, used the isocitrate dehydrogenase (IDH) gene mutation status as the classification molecular parameter to separate the GBM into three groups: GBM IDH-wild type, GBM IDH-mutant, and GBM NOS [37], with different prognosis [38]. In the past decade, GBM prognostic studies focused on mRNA or PCG as a result of the development of sequence technology and The Cancer Genome Atlas (TCGA) database. Chen et al. selected a gene expression signature score (GGESS) by incorporating ten glycolytic genes significantly correlated with patient survival and verified that the PCG signature could independently predict prognosis and response to chemotherapy of GBM patients [39]. According to Chinese Glioma Genome Atlas (CGGA) RNA sequencing database and TCGA DNA methylation, another study established a gene signature comprising eight differentially expressed genes affected by DNA methylation and validated its prognostic value for GBM patients [40]. A minimal multigene signature that correlated with patient survival and effectively separated the proneural and mesenchymal glioblastoma subtypes was developed from two patient-derived novel primary cell culture models (MTA10 and KW10) [41].
Recently, emerging evidence suggests that lncRNA play a vital role in cancer occurrence and development, such as regulating gene transcription [42] and post transcriptional processing of mRNA [43], participating in chromatin remodeling [44]. Subsequently, a great deal of lncRNAs have been shown to be closely associated with the survival of patients in different cancer types, indicating its prognostic prediction role. For GBM patients, some researchers identified a six-lncRNA signature associated with the overall survival by analyzing lncRNA expression profiling in 213 GBM tumors from TCGA [45]. An immune-related six-lncRNA signature was found by performing a genome-wide analysis of lncRNA expression profiles form 419 GBM patients and demonstrated its ability to stratify patients into high- and low-risk groups with significantly different survival [46]. All these above mentioned studies highlighted that it is feasible to mine the reliable and readily available expression profiles from TCGA database in GBM prognostic PCG/lncRNA marker studies. Moreover, a recent work found the dysregulated lncRNAs and mRNAs associated with acquired TMZ resistance in glioblastoma cells in vitro and may provide novel targets for GBM chemotherapy [47].
Therefore, in the present study, we combined the PCG expression profile with the tissue-specific lncRNA expression profile to explore a signature indicating the prognosis and therapy effectiveness of postoperative GBM patients. We obtained 233 postsurgical GBM patients with corresponding PCG, lncRNA expression profiles and clinical information as the study object. After summarized clinical characteristics, we found the median age of the postsurgical GBM patients was 60 and more common in men, almost consistent with most research reported [48,49,50]. Clinical treatment information of these 233 GBM patients provided convenience for our research on treatment response. Subsequently, we used two powerful bioinformatics analysis methods for identification of prognostic genes. Firstly, univariable cox regression analysis was performed and identified 707 genes that was significantly associated with the overall survival of GBM patients in the training dataset. Secondly, the random survival forest method further minimized the prognostic genes to 6 PCGs and 6 lncRNAs. Then we screened out a PCG-lncRNA signature with biggest AUC from 4095 combinations including different number of PCGs and/or lncRNAs, comprising six PCGs (EIF2AK3, EPRS, GALE, GUCY2C, MTHFD2, RNF212) and five lncRNAs (LINC00618, LINC02015, AC068888.1, CERNA1, CTD-2140B24.6), which separated patients into low-risk or high-risk group with different survival in the training and test dataset. The biggest AUC value of the PCG-lncRNA signature suggests it was better than any PCG alone signature or lncRNA alone signature. Multivariable Cox regression analysis verified the independence of the selected PCG-lncRNA signature from clinical factors like sex, age, KPS in predicting survival in postoperative GBM patients. As we mentioned, radiotherapy plus concomitant and maintenance TMZ chemotherapy after operation is the standard treatment for GBM patients, which means most postoperative GBM patients experienced TMZ-chemoradiation. Notably, the stratification analysis found that the PCG-lncRNA signature could further classify the TMZ-chemoradiation patients into low-risk or high-risk group with different survival, indicating the PCG-lncRNA signature could be helpful in predicting GBM treatment outcome, especially in TMZ-chemoradiation treated patients. Previous studies reported that age, MGMT promoter and IDH1 mutation were one of the main prognostic factors for GBM [45], so we compared the predictive ability of age, MGMT promoter and IDH1 mutation with that of the PCG-lncRNA signature, and the ROC analysis results confirmed the signature had a superior survival predictive power.
To further explore the characteristics of the prognostic PCGs and lncRNAs in the signature, we found EIF2AK3, EPRS, MTHFD2, RNF212, LINC02015, CTD-2140B24.6 were protected factors for GBM patients highly expressed these genes with a long survival time (univariable cox coefficient < 0), and the remaining genes (GALE, GUCY2C, LINC00618, AC068888.1, CERNA1) associated with short survival time were risk factors (univariable cox coefficient > 0) according to the univariable cox result in Table 2. Due to relevant functional research of the prognostic 11 genes are limited, we performed bioinformatics functional analyses including co-expression network analysis and pathway analysis. However, the biological roles of the selected genes in tumorigenesis are still not clear and should be investigated in further experimental studies.
There are some limitations in this work. Firstly, after rejecting missing data, only 6613 lncRNAs were included, which might neglect some potential lncRNAs. Secondly, only 233 patients were included in the analysis, thus the efficiency of the PCG-lncRNA signature should be confirmed in more GBM patients. Moreover, the molecular mechanisms how these prognostic genes or the PCG-lncRNA signature influence patients risk stratification and clinical treatment responses need to be explained.
Although the above shortcomings, this article still has advantages and novelty. Firstly, we used few genes which predict survival and construct a PCG-lncRNA signature with satisfactorily prognosis predictive power, giving the postoperative GBM patients and clinicians a potential signature to evaluate survival. Secondly, in the post-operative GBM patients, treated with radiotherapy or chemotherapy, we found the stratification power of the signature in TMZ-chemoradiation, which is helpful for clinical treatment guiding.
Conclusion
This is, to our knowledge, the first study investigating a correlation between the PCG-lncRNA signature and the survival in postoperative GBM patients. Our study strongly suggests that the PCG-lncRNA signature could serve as novel biomarkers for predicting prognosis and treatment outcome of postoperative GBM patients.
Abbreviations
- ROC:
-
receiver operating characteristic
- AUC:
-
area under the ROC curve
- PCG:
-
protein coding gene
- LncRNA:
-
long non-coding RNA
- OS:
-
overall survival
- GBM:
-
glioblastoma multiforme
- SD:
-
standard deviation
- TCGA:
-
The Cancer Genome Atlas
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19:v1–88.
Tamimi AF, Juweid M. Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma. Brisbane: Codon Publications; 2017.
Okada M, Miyake K, Tamiya T. Glioblastoma treatment in the elderly. Neurol Med Chir (Tokyo). 2017;57:667–76.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Curran WJ, Scott CB Jr., Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10.
Paravati AJ, Heron DE, Landsittel D, Flickinger JC, Mintz A, Chen YF, Huq MS. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of radiation therapy oncology group-recursive partitioning analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104:339–49.
Li J, Wang M, Won M, Shaw EG, Coughlin C, Curran WJ Jr., Mehta MP. Validation and simplification of the radiation therapy oncology group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:623–30.
Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–35.
Costa BM, Caeiro C, Guimaraes I, Martinho O, Jaraquemada T, Augusto I, Castro L, Osorio L, Linhares P, Honavar M, et al. Prognostic value of MGMT promoter methylation in glioblastoma patients treated with temozolomide-based chemoradiation: a Portuguese multicentre study. Oncol Rep. 2010;23:1655–62.
Rivera AL, Pelloski CE, Sulman E, Aldape K. Prognostic and predictive markers in glioma and other neuroepithelial tumors. Curr Probl Cancer. 2008;32:97–123.
Walid MS. Prognostic factors for long-term survival after glioblastoma. Perm J. 2008;12:45–8.
Li J, Liang R, Song C, Xiang Y, Liu Y. Prognostic significance of epidermal growth factor receptor expression in glioma patients. Onco Targets Ther. 2018;11:731–42.
Saadeh FS, Mahfouz R, Assi HI. EGFR as a clinical marker in glioblastomas and other gliomas. Int J Biol Markers. 2018;33:22–32.
Labussiere M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, Ciccarino P, Saulnier O, Paterra R, Marie Y, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200–6.
SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, LanXiao W, Fei Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103:269–73.
Tripathy K, Das B, Singh AK, Misra A, Misra S, Misra SS. Prognostic significance of epidermal growth factor receptor in patients of glioblastoma multiforme. J Clin Diagn Res. 2017;11:EC05–8.
Chen JR, Yao Y, Xu HZ, Qin ZY. Isocitrate dehydrogenase (IDH)1/2 mutations as prognostic markers in patients with glioblastomas. Medicine (Baltimore). 2016;95:e2583.
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–4.
Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus. 2015;38:E4.
Zhang Y, Xu J, Zhu X. A 63 signature genes prediction system is effective for glioblastoma prognosis. Int J Mol Med. 2018;41:2070–8.
Marko NF, Toms SA, Barnett GH, Weil R. Genomic expression patterns distinguish long-term from short-term glioblastoma survivors: a preliminary feasibility study. Genomics. 2008;91:395–406.
Reon BJ, Anaya J, Zhang Y, Mandell J, Purow B, Abounader R, Dutta A. Expression of lncRNAs in low-grade gliomas and glioblastoma multiforme: an in silico analysis. PLoS Med. 2016;13:e1002192.
Li J, Han L, Roebuck P, Diao L, Liu L, Yuan Y, Weinstein JN, Liang H. TANRIC: an interactive open platform to explore the function of lncRNAs in cancer. Can Res. 2015;75:3728.
Guo JC, Wu Y, Chen Y, Pan F, Wu ZY, Zhang JS, Wu JY, Xu XE, Zhao JM, Li EM, et al. Protein-coding genes combined with long noncoding RNA as a novel transcriptome molecular staging model to predict the survival of patients with esophageal squamous cell carcinoma. Cancer Commun (Lond). 2018;38:4.
Xu J, Li Y, Lu J, Pan T, Ding N, Wang Z, Shao T, Zhang J, Wang L, Li X. The mRNA related ceRNA-ceRNA landscape and significance across 20 major cancer types. Nucleic Acids Res. 2015;43:8169–82.
Becker RA, Chambers JM, Wilks AR. The new S language. Biometrics. 1988;45:935.
Ishwaran H, Lu M. Standard errors and confidence intervals for variable importance in random forest regression, classification, and survival. Stat Med. 2018. https://doi.org/10.1002/sim.7803.
Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50:1–23.
Li J, Chen Z, Tian L, Zhou C, He MY, Gao Y, Wang S, Zhou F, Shi S, Feng X, et al. LncRNA profile study reveals a three-lncRNA signature associated with the survival of patients with oesophageal squamous cell carcinoma. Gut. 2014;63:1700–10.
Cao HH, Zhang SY, Shen JH, Wu ZY, Wu JY, Wang SH, Li EM, Xu LY. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget. 2015;6:5435–48.
Zhou M, Guo M, He D, Wang X, Cui Y, Yang H, Hao D, Sun J. A potential signature of eight long non-coding RNAs predicts survival in patients with non-small cell lung cancer. J Transl Med. 2015;13:231.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Li C, Li X, Miao Y, Wang Q, Jiang W, Xu C, Li J, Han J, Zhang F, Gong B, et al. SubpathwayMiner: a software package for flexible identification of pathways. Nucleic Acids Res. 2009;37:e131.
Tse LA, Dai J, Chen M, Liu Y, Zhang H, Wong TW, Leung CC, Kromhout H, Meijer E, Liu S, et al. Prediction models and risk assessment for silicosis using a retrospective cohort study among workers exposed to silica in China. Sci Rep. 2015;5:11059.
Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Martinez-Garcia M, Alvarez-Linera J, Carrato C, Ley L, Luque R, Maldonado X, Martinez-Aguillo M, Navarro LM, Vaz-Salgado MA, Gil-Gil M. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin Transl Oncol. 2017;20(1):22–8.
Chen C, Shi Y, Li Y, He ZC, Zhou K, Zhang XN, Yang KD, Wu JR, Kung HF, Ping YF, et al. A glycolysis-based ten-gene signature correlates with the clinical outcome, molecular subtype and IDH1 mutation in glioblastoma. J Genet Genomics. 2017;44:519–30.
Wang W, Zhao Z, Wu F, Wang H, Wang J, Lan Q, Zhao J. Bioinformatic analysis of gene expression and methylation regulation in glioblastoma. J Neurooncol. 2018;136:495–503.
Sharma A, Bendre A, Mondal A, Muzumdar D, Goel N, Shiras A. Angiogenic gene signature derived from subtype specific cell models segregate proneural and mesenchymal glioblastoma. Front Oncol. 2017;7:146.
Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol. 2006;7:612–6.
Wang K, Sun T, Li N, Wang Y, Wang JX, Zhou LY, Long B, Liu CY, Liu F, Li PF. MDRL lncRNA regulates the processing of miR-484 primary transcript by targeting miR-361. PLoS Genet. 2014;10:e1004467.
Sun W, Yang Y, Xu C, Guo J. Regulatory mechanisms of long noncoding RNAs on gene expression in cancers. Cancer Genet. 2017;216–217:105–10.
Zhang XQ, Sun S, Lam KF, Kiang KM, Pu JK, Ho AS, Lui WM, Fung CF, Wong TS, Leung GK. A long non-coding RNA signature in glioblastoma multiforme predicts survival. Neurobiol Dis. 2013;58:123–31.
Zhou M, Zhang Z, Zhao H, Bao S, Cheng L, Sun J. An immune-related six-lncRNA signature to improve prognosis prediction of glioblastoma multiforme. Mol Neurobiol. 2018;55:3684–97.
Zeng H, Xu N, Liu Y, Liu B, Yang Z, Fu Z, Lian C, Guo H. Genomic profiling of long non-coding RNA and mRNA expression associated with acquired temozolomide resistance in glioblastoma cells. Int J Oncol. 2017;51:445–55.
Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104:2798–806.
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):1–56.
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–96.
Authors’ contributions
The authors contributed in the following way: Gao WZ, Guo LM, Xu TQ: data collection, data analysis, interpretation and drafting; Jia F, Yin YH: study design, study supervision and final approval of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully thank the academic editor and the anonymous reviewers for their insightful comments and suggestions to improve this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data generated or analysed during this study are available from the corresponding author upon reasonable request.
Additional material can be mentioned in the link: https://pan.baidu.com/s/1TKcsqUHUGxN0Y9jlSRVQ_A.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number 81471855 and 81701201).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
PCGs and lncRNAs of Univariate Cox regression analysis (P < 0.05) in the training set (n = 76).
Additional file 2: Table S2.
The 4095 signatures composed of different PCGs and lncRNAs in the training and test dataset.
Additional file 3: Table S3.
2328 genes co-expressed with the 11 molecular markers in the PCG-lncRNA signature (Pearson correlation coefficient > 0.4, P < 0.05, Entire group, n = 153).
Additional file 4: Table S4.
Pathway analysis of genes co-expressed with the prognostic PCGs or lncRNA in the signature by SubpathwayMiner.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gao, WZ., Guo, LM., Xu, TQ. et al. Identification of a multidimensional transcriptome signature for survival prediction of postoperative glioblastoma multiforme patients. J Transl Med 16, 368 (2018). https://doi.org/10.1186/s12967-018-1744-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-018-1744-8