Abstract
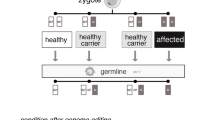
Cancer survivors can carry germline mutations that will be transmitted to their progeny. Today, many of these mutations have been identified and can be tracked. With the recent development of genome-editing technologies and CRISPR (clustered regularly interspaced short palindromic repeats), the possibility of genetically modifying the human germline—gametes and embryos—has never been closer. This perspective has sparked a controversy within the scientific community with reactions ranging from calls for a ban on germline modification to cautious approval of further research. This Editorial analyzes the possible adoption of CRISPR-based germline engineering to prevent the spread of cancer predispositions in the human population. We discuss whether the genomic edition of human sperm and eggs would contribute to rectifying or altering the heritable genome. We anticipate the emergence of a new form of liberal eugenics fueled by a logic of offer and demand from stakeholders such as cancer survivors and their relatives and offspring, but also from fertility clinics, biotech firms, insurers, and clinicians. From a regulatory perspective, validating the clinical safety and utility of CRISPR-based germline engineering is an essential step. However, with time, gradually perfecting the technology and assessing the economic benefits for stakeholders could soften society’s resistance and align opinions in support of genomic decontamination of human germlines. This progressive shift would be justified in the name of cancer prevention as well as a moral obligation to facilitate the conception of cancer-free children at a cost that is acceptable to individuals and health systems.
Similar content being viewed by others
Change history
30 December 2018
The above-referenced article was published in the issue with an incorrect article type. It was listed as a Commentary article, but should have been listed as an Editorial.
References
Nekhulyudov L, Walker R, Ziebell R, Rabin B, Nutt S, Chubak J. Cancer survivors’ experiences with insurance, finances, and employment: results from a multisite study. J Cancer Surviv. 2016;10:1104–1111.
Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421.
Kandoth C, McLellan MD, Vandin F. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339.
Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339:1546–1558.
Topol E. Individualized medicine from pre-womb to tomb. Cell. 2014;157:241–253.
Nakayama T, et al. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843.
Doudna J, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096.
Ledford H. Riding the CRISPR wave. Nature. 2016;531:156–159.
Hu X, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111 11461–11466.
Hammond A, Galizi R, Kyrou K, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34:78–83.
Zhang H, Zhang J, Wei P, et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J. 2014;12:797–807.
Jia H, Wang N. Targeted genome editing of sweet orange using Cas9/sgRNA. PLoS One. 2014;9:e93806.
Wang Z. Genome engineering in cattle: recent technological advancements. Chromosome Res. 2015;23:17–29.
Reardon S. The CRISPR zoo. Nature. 2016;531:160–163.
Shi J, Wang E, Milazzo JP, et al. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol. 2015;33:661–667.
Yin H, Xue W, Chen S, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551–553.
Long C, McAnally JR, Shelton JM, et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184–1188.
Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T. Precise correction of the dystrophin gene in Duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep. 2015;4:143–154.
Tabebordbar M, Zhu K, Cheng JKW, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411.
Wu Y, Liang D, Wang Y, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13:659–662.
Chen J-R, Tang ZH, Zheng J, et al. Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016;23:1347–1357.
Xie F, Tang ZH, Zheng J, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551–553.
Shalem O, Sanjana NE, Hartenian E, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87.
Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658.
Reardon S. Gene editing wave hits clinic. Nature. 2015;527:146–147.
Cyranoski D. First trial of CRISPR in people. Nature. 2016;535:476–477.
Kaiser J. First proposed human test of CRISPR passes initial safety review. Science, June 21, 2016.
Mulder C, Zheng Y, Jan SZ, et al. Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update. 2016;5:561–573.
Chapman KM, Medrano GA, Jaichander P, et al. Targeted germline modifications in rats using CRISPR/Cas9 and spermatogonial stem cells. Cell Rep. 2015;10:1828–1835.
Sato T, Sakuma T, Yokonishi T, et al. Genome editing in mouse spermatogonial stem cell lines using TALEN and double-nicking CRISPR/Cas9. Stem Cell Rep. 2015;5:75–82.
Vassena R, Heindryckx B, Peco R, et al. Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update. 2016;22:411–419.
Gomy I, Del Pilar Estevez Diz M. Hereditary cancer risk assessment: insights and perspectives for Next-Generation Sequencing era. Genet Mol Biol. 2016;39:184–188.
Kraus C, Rau T, Lux P, et al. Comprehensive screening for mutations associated with colorectal cancer in unselected cases reveals penetrant and nonpenetrant mutations. Int J Cancer. 2015;136:E559–E568.
Nelson HD, Fu R, Goddard K, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Evidence Syntheses, No. 101. Rockville, MD: US Agency for Healthcare Research and Quality; 2013.
Pharoah PD, Antoniou A, Bobrow M, et al. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet. 2002;31:33–36.
Houlston RS, Peto F. The search for low-penetrance cancer susceptibility alleles. Oncogene. 2004;23:6471–6476.
Newson A, Leonard S, Hall A, Gaff CL. Known unknowns: building an ethics of uncertainty into genomic medicine. BMC Med Genom. 2016;9:57.
Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667.
Lynce F, Isaacs C. How Far Do We Go With Genetic Evaluation? Gene, Panel, and Tumor Testing. ASCO Educational Book. 2016;E72.
Kaiser J, Normile D. Embryo engineering study splits scientific community. Science. 2015;348:486–487.
Araki M, Ishii T. International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol. 2014;12:108.
Ledford H. The landscape for human genome editing. Nature. 2015;526:310–311.
Council of Europe, Convention on Human Rights and Biomedecine, article 13. https://rm.coe.int/CoERMPublicCommonSearchServices/DisplayDCTMContent?documentId=090000168049034a (2015).
The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015 No. 572. http://www.legislation.gov.uk/ukdsi/2015/9780111125816/contents.
Liang P, Xu Y, Zhang X, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–372.
The National Academies of Science, Engineering and Medicine. International Summit on Human Gene Editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a. Published December 3, 2015.
Reardon S. Global summit reveals divergent views on human gene editing. Nature. 2015;528:173.
Collins FS. Statement on NIH funding of research using gene-editing technologies in human embryos. National Institutes of Health. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos. Published 2015.
DiCarlo JE, Chavez A, Dietz SL, Esvelt KM, Church GM. Safeguarding CRISPR-Cas9 gene drives in yeast. Nat Biotechnol. 2015;33:1250–1255.
Akbari OS, Bellen HJ, Bier E, et al. Biosafety. Safeguarding gene drive experiments in the laboratory. Science. 2015;349:927–929.
Newson AJ, Wrigley A. Identifying key developments, issues and questions relating to techniques of genome editing with engineered nucleases. Background paper. London: Nuffield Council on Bioethics. http://nuffieldbioethics.org/wp-content/uploads/Genome-Editing-Briefing-Paper-Newson-Wrigley.pdf (2015).
Brice P. First UK birth following PGD for hereditary breast cancer PHG Foundation, January 9, 2009. http://www.phgfoundation.org/news/4445/. Published 2016.
Gelbaya TA. Short and long-term risks to women who conceive through in vitro fertilization. Hum Fertil. 2010;13:19–27.
Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19:330–353.
Kaimal A, Norton M, Kuppermann M. Prenatal testing in the genomic age: clinical outcomes, quality of life and costs. Obstet Gynecol. 2015;126:737–746.
Verhoef TI, Hill M, Drury S. Non-invasive prenatal diagnosis (NIPD) for single gene disorders: cost analysis of NIPD and invasive testing pathways. Prenat Diagn. 2016;36:636–642.
Benett J, Chitty L, Lewis C. Non-invasive prenatal diagnosis for BRCA mutations—a qualitative pilot study of health professionals’ views. J Genet Counsel. 2016;25:198–207.
Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff. 2013;32:1143–1152.
Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332–338.
deBoer AG, Taskila T, Ojajärvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301:753–762.
Guy GP Jr., Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31:3749–3957.
Yabroff KR, Dowling EC, Guy GP Jr., et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–267.
Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34:1732–1740.
Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:238–239.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986.
NIH, National Cancer Institute. Cancer prevalence and cost of care projections. https://costprojections.cancer.gov/expenditures.html#. Published 2016.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–1174.
Lyman G. Counting the costs of cancer care. Lancet Oncol. 2013;14:1142–1143.
Tefferi A, Kantarjian H, Rajkumar V, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90:996–1000.
US Census Bureau. Families and households. http://www.census.gov/hhes/families/index.html. Accessed January 1, 2017.
McCarthy M. US oncologists call for government regulation to curb drug price rises. BMJ. 2015;351:h4063.
NIH, National Human Genome Research Institute. DNA Sequencing Costs: Data. https://www.genome.gov/27541954/dna-sequencing-costs-data/dna-sequencing-costs-data/. Accessed January 1, 2017.
ThermoFisher Scientific. GeneArt Platinum Cas9 Nuclease & Lipofectamine CRISPRMAX. https://www.thermofisher.com/fr/fr/home/life-science/genome-editing/geneart-crispr/crispr-protein.html. Accessed January 1, 2017.
Boettcher M, McManus M. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell. 2015;58:575–585.
Unniyampurath U, Pilankatta R, Krishnan M. RNA interference in the age of CRISPR: will CRISPR interfere with RNAi? Int J Mol Sci. 2016;17:291.
Schumpeter JA. Capitalism, Socialism and Democracy. London: Routledge; 1942:82–83.
Subramanian S, Tangka FK, Hoover S, et al., Recommendations from the international colorectal cancer screening network on the evaluation of the cost of screening programs. J Public Health Manag Pract. 2015;22:461–465.
Ouakrim D, Boussioutas A, Lockett T, Hopper JL, Jenkins MA. Cost-effectiveness of family history-based colorectal cancer screening in Australia. BMC Cancer. 2014;14:261.
Pil L, Fobelets M, Putman K, et al. Cost-effectiveness and budget impact analysis of a population-based screening program for colorectal cancer. Eur J Int Med. 2016;32:72–78.
President’s Council of Advisors on Science and Technology. Priorities for Personalized Medicine. Office of Science and Technology, US, 2008. https://www.whitehouse.gov/files/documents/ostp/PCAST/pcast_report_v2.pdf.
Towse A, Garrison L. Value Assessment in Precision Cancer Medicine. J Cancer Policy. 2016. https://doi.org/10.1016/j.jcpo.2016.09.003.
Harris L, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150.
National Institute for Health and Care Excellence. Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat. https://www.nice.org.uk/guidance/DG10. Accessed January 1, 2017.
Katz G, Romano O, Foa C, et al. Economic impact of gene expression profiling in patients with early-stage breast cancer in France. PLoS One. 2015;10:1–15.
Lander E. Brave new genome. N Engl J Med. 2015;373:1.
Katz G, Schweitzer S. Implications of genetic testing for health policy. Yale J Health Policy Law Ethics. 2010;10:89–134.
Joly Y, Burton H, Knoppers BM, et al. Life Insurance: genomic stratification and risk classification. Eur J Hum Genet. 2014;22:575–579.
Withrock I, Anderson S, Jefferson M, et al. Genetic diseases conferring resistance to infectious diseases. Genes Dis. 2015;2:247–254.
Quételet A. Anthropométrie ou mesure des différentes facultés de l’homme (1870), Nabu Press, 2014.
Macdonald AS. Genetic factors in life insurance. Actuarial basis. In: Encyclopedia of Life Science. Chichester, UK: Wiley; 2009.
Van Hoyweghen I, Rebert L. Your genes in insurance: from genetic discrimination to genomics solidarity. Pers Med. 2012;9:871–877.
Van Erp PBG, Bloomer G, Wilkinson R, Wiedenheft B. The history and market impact of CRISPR RNA-guided nucleases. Curr Opin Virol. 2015;12:85–90.
Editing Humanity. The Economist, August 22–28, 2015.
Egelie K, Graff G, Strand S, Johansen B. The emerging patent landscape of CRISPR-Cas gene editing technology. Nat Biotechnol. 2016;34:1025–1031.
Zoll M, Mertes H, Gupta J, Corporate giants provide fertility benefits: have they got it wrong? Eur J Obstet Gynecol Reprod Biol. 2015;195:A1–A2.
Silver AJ, Larson JL, Silver MJ. Carrier screening is a deficient strategy for determining sperm donor eligibility and reducing risk of disease in recipient children. Genet Test Mol Biomarkers. 2016;20:276–284.
Rojahn Y. Genetic screening can uncover risky matches at the sperm bank. MIT Technology Review, November 20, 2012.
Wojcicki A, Avey L, Mountain JL, McPherson JM, Tung JYH. Gamete donor selection based on genetic calculations. US 2010/0145981 A1, paria. [0014]. 2010. US Patent and Trademark Office. Notice of Allowance in Relation to US Patent Application Serial No. 12/592950.
Sterckx S, Cockbain J, Howard H, Borry P. «I prefer a child with…»: designer babies, another controversial patent in the arena of direct-to-consumer genomics. Genet Med. 2013;15:923–924.
Regalado A. Engineering the perfect baby. MIT Technology Review, March 5, 2015.
Hildt E. Human germline intervention—think first. Front Genet. 2016;7:81.
Sugarman J. Ethics and germline gene editing, EMBO Rep. 2015;16:879–880.
Blendon R, et al. The public and the gene-editing revolution. N Eng J Med. 2016;374:1406–1411.
STAT-Harvard T.H. Chan School of Public Health. The public and genetic editing, testing and therapy. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/94/2016/01/STAT-Harvard-Poll-Jan-2016-Genetic-Technology.pdf (2016).
Pew Research Center. US public wary of biomedical technologies to “enhance” human abilities, July 26, 2016. http://www.pewinternet.org/2016/07/26/u-s-public-wary-of-biomedical-technologies-to-enhance-human-abilities/.
Hayden EC. Tomorrow’s children. Nature. 2016;530:402–405.
Hayden EC. Tomorrow’s children. Nature. 2016;530:402–405.
Häyry M. There is a difference between selecting a deaf embryo and deafening a hearing child. J Med Ethics. 2004;30:510–512.
Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48:977–988.
Bruno MA. A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open. 2011;1:e000039.
Levine S. The changing terrains in medical sociology: emergent concern with the quality of life. J Health Soc Behav. 1987;28:1–6.
Savulescu J, Kahane G. The moral obligation to create children with the best chance of the best life. Bioethics. 2009;23:274–290.
Nietzsche, Fragments posthumes 1888–1889, JC, tome XIV, Oeuvres philosophiques complètes, trad. Hemery, Paris, Gallimard (1977).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katz, G., Pitts, P.J. Implications of CRISPR-Based Germline Engineering for Cancer Survivors. Ther Innov Regul Sci 51, 672–682 (2017). https://doi.org/10.1177/2168479017723401
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479017723401