Abstract
Background
Raltegravir is an integrase strand transfer inhibitor indicated in combination with other anti-retroviral medicinal products for the treatment of HIV-1 infection, given twice daily. Although a BCS class II compound, raltegravir exhibits low colonic absorption, thus making development of modified release formulations challenging. It was hypothesized that a gastroretentive (GR) formulation would increase trough (C24 h) concentrations of raltegravir, hence being amenable to a once-daily (QD) regimen.
Methods
Iterative prototype GR formulations were developed in monolithic, bilayer, and trilayer tablet designs. Four phase I studies in healthy subjects were conducted to provide proof of formulation concept.
Results
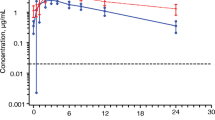
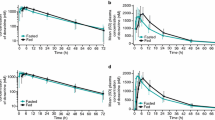
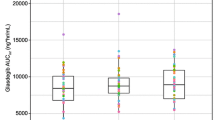
Raltegravir C24 h was increased by a GR formulation with scintigraphy data supporting gastric retention. Single-dose exposures from a trilayer tablet administered in the morning with a high-fat meal resulted in acceptable C24 h values. However, C24 h values for evening dosing with a high-fat meal did not meet the success criteria for QD administration. Raltegravir C24 h and area under the curve (AUC) values after AM dosing with a low-fat meal were significantly lower than after dosing with a moderate-fat meal. Skipping, delaying, or giving a low-fat, low-calorie lunch after dosing with a high-calorie breakfast also resulted in an unacceptable decline in C24 h values. The requirements for consistent product performance under varying conditions of diet, timing of dosing, and dose were not favorable when given as GR tablets.
Conclusions
The findings from these studies offer valuable insights into modifying the absorption of candidate drugs with limiting colonic permeability and solubility characteristics and the interplay between meal, dose timing, and GR formulation performance.
Similar content being viewed by others
References
Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J Control Release. 2003;90:143–162.
Hwang SJ, Park H, Park K. Gastric retentive drug delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243–284.
Moes AJ. Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst. 1993;10:143–195.
Streubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery systems. Expert Opin Drug Deliv. 2006;3:217–233.
Chen C, Han C-H, Sweeney M, Cowles VE. Pharmacokinetics, efficacy, and tolerability of a once-daily gastroretentive dosage form of gabapentin for the treatment of postherpetic neuralgia. J Pharm Sci. 2013;102:1155–1164.
Berner B, Cowles VE. Case studies in swelling polymeric gastric retentive tablets. Expert Opin Drug Deliv. 2006;3:541–548.
Gusler G, Gorsline J, Levy G, et al. Pharmacokinetics of metformin gastric-retentive tablets in healthy volunteers. J Clin Pharmacol. 2001;41:655.
Gusler G, Berner B. Metformin (GR™) Gastric Retentive Tablets: GI Transit and Pharmacokinetics in Healthy Volunteers. San Francisco: Millenial World Congress of Pharmaceutical Sciences; 2000; 2–5019.
Hoffman A, Stepensky D, Lavy E, Eyal S, Klausner E, Friedman M. Pharmacokinetic and pharmacodynamics aspects of gastroretentive dosage forms. Int J Pharm. 2004;277:141–153.
Tannergren C, Bergendal A, Lennernäs H, Abrahamsson B. Toward an increased understanding of the barriers to colonic drug absorption in humans: implications for early controlled release candidate assessment. Mol Pharm. 2009;6:60–73.
Summa V, Petrocchi A, Bonelli F, et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem. 2008;51:5843–555.
Brainard DM, Wenning LA, Stone JA, Wagner JA, Iwamoto M. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J Clin Pharmacol. 2011;51:1376–1402.
Walji AM, Sanchez RI, Clas SD, et al. Discovery of MK-8970: an acetal carbonate prodrug of raltegravir with enhanced colonic absorption. ChemMedChem. 2015;10:245–252.
Gupta P, Patel S, Lin W, et al. Gastroretentive Dosage Form for Once-Daily (QD) Dosing of a Compound with a Narrow Absorption Window. Controlled Release Society (2015).
Rizk ML, Hang Y, Luo WL, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012;56:3101–3106.
Ananworanich J, Gorowara M, Avihingsanon A, et al. Pharmacokinetics of and short-term virologic response to low-dose 400-milligram once-daily raltegravir maintenance therapy. Antimicrob Agents Chemother. 2012;56:1892–1899.
Coupe AJ, Davis SS, Evans DF, Wilding IR. Nocturnal scintigraphic imaging to investigate the gastrointestinal transit of dosage forms. J Control Release. 1992;20:155–162.
Merschman SA, Vallano PT, Wenning LA, Matuszewski BK, Woolf EJ. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:15–19.
Eron JJ Jr., Rockstroh JK, Reynes J, et al.; QDMRK Investigators. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11:907–915.
Timmermans J, Moes AJ. The cutoff size for gastric emptying of dosage forms. J Pharm Sci. 1993;82:854.
Brainard DM, Friedman EJ, Jin B, et al. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J Clin Pharmacol. 2011;51:422–427.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna, R., Rizk, M.L., Larson, P.J. et al. Novel Gastroretentive Controlled Release Formulations for Once-Daily Administration: Assessment of Clinical Feasibility and Formulation Concept for Raltegravir. Ther Innov Regul Sci 50, 777–790 (2016). https://doi.org/10.1177/2168479016657130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479016657130