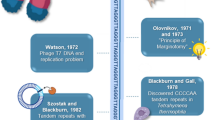
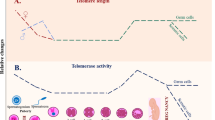
Abstract
In modern society, fertility problems and demand of treatment seem to be on the rise, which led to an increased interest in research regarding human reproduction. Among these efforts, the study of the molecular senescence process has gain notorious popularity as aging is one of the most important variables involved in reproductive capacity and since the comprehension of telomere dynamics has become an important and influential theme. This new knowledge regarding the reproductive aging process is expected to offer new tools to understand the acquisition, maintenance, and loss of fertility potential. Therefore, this review seeks to clarify the relevance of molecular aging (evaluated by telomere shortening) in human reproduction, showing that it is a dynamic and variable process modulated according to the specific tissue and stage of development. As well, it is discussed how telomere status influence the development and progression of some fertility pathologies, the outcome of assisted reproductive treatments, and programming of aging in the offspring.
Similar content being viewed by others
References
Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. doi:10.1038/nrg1656
Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. FEBS J. 2013;280(14):3180–3193. doi:10.1111/febs. 12326
Lai TP, Wright WE, Shay JW. Comparison of telomere length measurement methods. Philos Trans R Soc B Biol Sci. 2018; 373(1741):20160451. doi:10.1098/rstb.2016.0451
Montpetit AJ, Alhareeri AA, Montpetit M, et al. Telomere length: a review of methods for measurement. Nurs Res. 2014;63(4): 289–299. doi:10.1097/NNR.0000000000000037
Chan SRWL, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359(1441):109–121. doi:10. 1098/rstb.2003.1370
Sukenik-Halevy R, Fejgin M, Kidron D, et al. Telomere aggregate formation in placenta specimens of pregnancies complicated with pre-eclampsia. Cancer Genet Cytogenet. 2009;195(1):27–30. doi: 10.1016/j.cancergencyto.2009.03.015
Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8(1):3–11. doi:10.18632/aging.100871
Victorelli S, Passos JF. Telomeres and cell senescence—size matters not. EBioMedicine. 2017;21:14–20. doi:10.1016/j.ebiom.
George K, Kamath MS. Fertility and age. J Hum Reprod Sci. 2010;3(3):121–123. doi:10.4103/0974-1208.74152
Harris ID, Fronczak C, Roth L, Meacham RB. Fertility and the aging male. Rev Urol. 2011;13(4):e184–e190. http://www.ncbi.nlm.nih.gov/pubmed/22232567.
Kocourkova J, Burcin B, Kucera T. Demographic relevancy of increased use of assisted reproduction in European countries. Reprod Health. 2014;11:37. doi:10.1186/1742-4755-11-37
Stephen EH, Chandra A, King RB. Supply of and demand for assisted reproductive technologies in the United States: clinic-and population-based data, 1995-2010. Fertil Steril. 2016; 105(2):451–458. doi:10.1016/j.fertnstert.2015.10.007
Aydos SE, Elhan AH, Tükün A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet. 2005;272(2):113–116. doi:10.1007/s00404-004-0690-2
Monaghan P, Eisenberg DTA, Harrington L, Nussey D. Under-standing diversity in telomere dynamics. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741):20160435. doi:10.1098/rstb.2016.0435
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–179. doi:10.1002/(SICI)1520-6408(1996)18:2<173:: AID-DVG10>3.0.CO;2-3
Jørgensen PB, Fedder J, Koelvraa S, Graakjaer J. Age-dependence of relative telomere length profiles during spermato-genesis in man. Maturitas. 2013;75(4):380–385. doi:10.1016/j.maturitas.2013.05.001
Reig-Viader R, Vila-Cejudo M, Vitelli V, et al. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis J. Biol Reprod. 2014;90(5):780–791. doi:10.1095/biolreprod.113.116954
Schrader M, Müller M, Schulze W, et al. Quantification of the expression level of the gene encoding the catalytic subunit of telomerase in testicular tissue specimens predicts successful sperm recovery. Hum Reprod. 2002;17(1):150–156. http://www.ncbi.nlm.nih.gov/pubmed/11756380.
Schrader M, Müller M, Heicappell R, Straub B, Miller K. Quantification of human telomerase RNA (hTR) and human telomerase reverse transcriptase (hTERT) mRNA in testicular tissue of infertile patients. Asian J Androl. 2001;3(4):263–270. http://www.ncbi.nlm.nih.gov/pubmed/11753470.
Schrader M, Müller M, Heicappell R, Krause H, Schulze W, Miller K. Telomerase activity and expression of telomerase sub-units in the testicular tissue of infertile patients. Fertil Steril. 2000;73(4):706–711. http://www.ncbi.nlm.nih.gov/pubmed/10731529.
Weikert S, Christoph F, Schulze W, et al. Testicular expression of survivin and human telomerase reverse transcriptase (hTERT) associated with spermatogenic function in infertile patients. Asian J Androl. 2006;8(1):95–100. doi:10.1111/j.1745-7262.2006. 00102.x
Reig-Viader R, Brieno-Enriquez MA, Khouriauli L, et al. Telo-meric repeat-containing RNA and telomerase in human fetal oocytes. Hum Reprod. 2013;28(2):414–422. doi:10.1093/hum-rep/des363
Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod. 2001;7(10):947–955. http://www.ncbi.nlm.nih.gov/pubmed/11574663.
Turner S, Wong HP, Rai J, Hartshorne GM. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod. 2010;16(9):685–694. doi:10.1093/molehr/gaq048
Treff NR, Su J, Taylor D, Scott RT Jr. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi:10.1371/journal.pgen.1002161
Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY). 2016;8(2):216–230. doi: 10.18632/aging.100891
Gielen M, Hageman G, Pachen D, Derom C, Vlietinck R, Zeegers MP. Placental telomere length decreases with gestational age and is influenced by parity: a study of third trimester live-born twins. Placenta. 2014;35(10):791–796. doi:10.1016/j.placenta.2014.05. 010
Menon R, Yu J, Basanta-Henry P, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7(2):e31136. doi:10.1371/journal.pone.0031136
Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? Sun K, ed. PLoS One. 2015;10(9):e0137188. doi:10.1371/journal.pone.0137188
Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–1130. doi:10.1016/j.cell.2013. 10.041
Mun˜oz-Espín D, Can˜amero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–1118. doi:10.1016/j.cell.2013.10.019
Chuprin A, Gal H, Biron-Shental T, et al. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. 2013;27(21):2356–2366. doi:10.1101/gad.227512.113
Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: a sign of premature senescence? J Matern Neonatal Med. 2016;29(8):1283–1288. doi:10.3109/14767058. 2015.1046045
Biron-Shental T, Sukenik Halevy R, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Telomeres are shorter in placental tro-phoblasts of pregnancies complicated with intrauterine growth restriction (IUGR). Early Hum Dev. 2010;86(7):451–456. doi: 10.1016/j.earlhumdev.2010.06.002
Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 2009;30(6):539–542. doi:10.1016/j.placenta.2009.03.005
Biron-Shental T, Sukenik-Halevy R, Sharon Y, et al. Short telo-meres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2010; 202(4):381.e1–381.e7. doi:10.1016/j.ajog.2010.01.036
Toutain J, Prochazkova-Carlotti M, Cappellen D, et al. Reduced placental telomere length during pregnancies complicated by intrauterine growth restriction. Tian X (Cindy), ed. PLoS One. 2013;8(1):e54013. doi:10.1371/journal.pone.0054013
Biron-Shental T, Sukenik-Halevy R, Sharon Y, Laish I, Fejgin MD, Amiel A. Telomere shortening in intra uterine growth restriction placentas. Early Hum Dev. 2014;90(9):465–469. doi: 10.1016/j.earlhumdev.2014.06.003
Sukenik-Halevy R, Amiel A, Kidron D, Liberman M, Ganor-Paz Y, Biron-Shental T. Telomere homeostasis in trophoblasts and in cord blood cells from pregnancies complicated with preeclampsia. Am J Obstet Gynecol. 2016;214(2):283.e1-283.e7. doi:10. 1016/j.ajog.2015.08.050
Biron-Shental T, Liberman M, Elbaz M, Laish I, Sharony R, Amiel A. Telomere homeostasis in placentas from pregnancies with uncontrolled diabetes. Placenta. 2016;44:13–18. doi:10. 1016/j.placenta.2016.05.009
Biron-Shental T, Sukenik-Halevy R, Naboani H, Liberman M, Kats R, Amiel A. Telomeres are shorter in placentas from pregnancies with uncontrolled diabetes. Placenta. 2015;36(2): 199–203. doi:10.1016/j.placenta.2014.11.011
Zinkovaá A, Marovaá D, Koperdaákovaá J, Mirchi TP, Korabecnaá M, Jirkovskaá M. Relative amount of telomeric sequences in terminal villi does not differ between normal term placentas and placentas from patients with well-controlled type 1 diabetes mellitus. Placenta. 2017;55:1–4. doi:10.1016/j.placenta.2017.04.016
Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287(4):803–807. doi:10. 1007/s00404-012-2632-8
Shuyuan L, Changjun Z, Haiying P, et al. Association study of telomere length with idiopathic male infertility. Yi Chuan. 2015; 37(11):1137–1142. doi:10.16288/j.yczz.15-267
Reig-Viader R, Capilla L, Vila-Cejudo M, et al. Telomere home-ostasis is compromised in spermatocytes from patients with idio-pathic infertility. Fertil Steril. 2014;102(3):728.e1–738.e1. doi: 10.1016/j.fertnstert.2014.06.005
Biron-Shental T, Wiser A, Hershko-Klement A, Markovitch O, Amiel A, Berkovitch A. Sub-fertile sperm cells exemplify telo-mere dysfunction. J Assist Reprod Genet. 2018;35(1):143–148. doi:10.1007/s10815-017-1029-9
Antunes DMF, Kalmbach KH, Wang F, et al. A single-cell assay for telomere DNA content shows increasing telomere length het-erogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015; 32(11):1685–1690. doi:10.1007/s10815-015-0574-3
Ferlin A, Rampazzo E, Rocca MS, et al. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod. 2013;28(12):3370–3376. doi:10.1093/humrep/det392
Yang Q, Zhang N, Zhao F, et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online. 2015;31(1):44–50. doi:10.1016/j.rbmo.2015.02.016
Yang Q, Zhao F, Dai S, et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum Reprod. 2015;30(8):1876–1881. doi:10.1093/humrep/dev144
Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31(6):1158–1163. doi:10.1093/humrep/dew061
Thilagavathi J, Mishra SS, Kumar M, et al. Analysis of telomere length in couples experiencing idiopathic recurrent pregnancy loss. J Assist Reprod Genet. 2013;30(6):793–798. doi:10.1007/s10815-013-9993-1
Mishra S, Kumar R, Malhotra N, Singh N, Dada R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J Methodol. 2016;6(2):163. doi:10.5662/wjm.v6.i2.163
Santiso R, Tamayo M, Gosaálvez J, Meseguer M, Garrido N, Fernaández JL. Swim-up procedure selects spermatozoa with longer telomere length. Mutat Res Mol Mech Mutagen. 2010; 688(1-2):88–90. doi:10.1016/j.mrfmmm.2010.03.003
Zhao F, Yang Q, Shi S, Luo X, Sun Y. Semen preparation methods and sperm telomere length: density gradient centrifugation versus the swim up procedure. Sci Rep. 2016;6:39051. doi:10. 1038/srep39051
Arias-Sosa LA, Acosta ID, Lucena-Quevedo E, Moreno-Ortiz H, Esteban-Pérez C, Forero-Castro M. Genetic and epigenetic varia-tions associated with idiopathic recurrent pregnancy loss. J Assist Reprod Genet. 2018;35(3):355–366. doi:10.1007/s10815-017-1108-y
Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24(5):1206–1211. doi:10.1093/humrep/dep007
Bhaumik P, Bhattacharya M, Ghosh P, Ghosh S, Kumar Dey S. Telomere length analysis in Down syndrome birth. Mech Ageing Dev. 2017;164:20–26. doi:10.1016/j.mad.2017.03.006
Ghosh S, Feingold E, Chakraborty S, Dey SK. Telomere length is associated with types of chromosome 21 nondisjunction: a new insight into the maternal age effect on Down syndrome birth. Hum Genet. 2010;127(4):403–409. doi:10.1007/s00439-009-0785-8
Albizua I, Rambo-Martin BL, Allen EG, He W, Amin AS, Sherman SL. Association between telomere length and chromosome 21 nondisjunction in the oocyte. Hum Genet. 2015;134(11-12): 1263–1270. doi:10.1007/s00439-015-1603-0
Ray A, Hong CS, Feingold E, et al. Maternal telomere length and risk of down syndrome: epidemiological impact of smokeless chewing tobacco and oral contraceptive on segregation of chromosome 21. Public Health Genomics. 2015;19(1):11–18. doi:10.1159/000439245
Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94(12):4835–4843. doi:10.1210/jc.2008-2269
Xu X, Chen X, Zhang X, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2016;32(1):201–207. doi:10.1093/humrep/dew283
Li Q, Du J, Feng R, et al. A possible new mechanism in the pathophysiology of polycystic ovary syndrome (PCOS): the discovery that leukocyte telomere length is strongly associated with PCOS. J Clin Endocrinol Metab. 2014;99(2):E234–E240. doi:10. 1210/jc.2013-3685
Pedroso DCC, Miranda-Furtado CL, Kogure GS, et al. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil Steril. 2015;103(2):542.e1–547.e2. doi:10. 1016/j.fertnstert.2014.10.035
Wei D, Xie J, Yin B, et al. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 2017;34(7):861–866. doi:10. 1007/s10815-017-0945-z
Li Y, Deng B, Ouyang N, Yuan P, Zheng L, Wang W. Telomere length is short in PCOS and oral contraceptive does not affect the telomerase activity in granulosa cells of patients with PCOS. J Assist Reprod Genet. 2017;34(7):849–859. doi:10.1007/ s10815-017-0929-z
Czamanski-Cohen J, Sarid O, Cwikel J, et al. Cell-free DNA and telomere length among women undergoing in vitro fertilization treatment. J Assist Reprod Genet. 2015;32(11):1697–1703. doi: 10.1007/s10815-015-0581-4
Wang W, Chen H, Li R, et al. Telomerase activity is more significant for predicting the outcome of IVF treatment than telo-mere length in granulosa cells. Reproduction. 2014;147(5): 649–657. doi:10.1530/REP-13-0223
Chen H, Wang W, Mo Y, et al. Women with high telomerase activity in luteinised granulosa cells have a higher pregnancy rate during in vitro fertilisation treatment. J Assist Reprod Genet. 2011;28(9):797–807. doi:10.1007/s10815-011-9600-2
Cheng EH, Chen SU, Lee TH, et al. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28(4):929–936. doi:10.1093/humrep/ det004
Njajou OT, Cawthon RM, Damcott CM, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12135–12139. doi:10.1073/ pnas.0702703104
Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Roos G. Large-scale parent-child comparison confirms a strong paternal influence on telomere length. Eur J Hum Genet. 2010;18(3): 385–389. doi:10.1038/ejhg.2009.178
Nordfjäll K, Larefalk A, Lindgren P, Holmberg D, Roos G. Telomere length and heredity: indications of paternal inheritance. Proc Natl Acad Sci U S A. 2005;102(45):16374–16378. doi:10. 1073/pnas.0501724102
Kimura M, Cherkas LF, Kato BS, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. doi:10.1371/journal.pgen.0040037
Eisenberg DTA, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci U S A. 2012;109(26):10251–10256. doi:10.1073/pnas.1202092109
Aston KI, Hunt SC, Susser E, et al. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18(11):517–522. doi:10.1093/molehr/gas028
Turner S, Hartshorne GM. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod. 2013;19(8):510–518. doi:10.1093/molehr/gat021
Eisenberg DTA, Kuzawa CW. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Philos Trans R Soc Lond B Biol Sci. 2018; 373(1741):20160442. doi:10.1098/rstb.2016.0442
Entringer S, de Punder K, Buss C, Wadhwa PD. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741):20170151. doi: 10.1098/rstb.2017.0151
Entringer S, Epel ES, Lin J, et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2013;208(2):134.e1-134.e7. doi:10. 1016/j.ajog.2012.11.033
Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrau-terine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–E518. doi:10.1073/pnas.1107759108
Salihu HM, King LM, Nwoga C, et al. Association between maternal-perceived psychological stress and fetal telomere length. South Med J. 2016;109(12):767–772. doi:10.14423/SMJ. 0000000000000567
Marchetto NM, Glynn RA, Ferry ML, et al. Prenatal stress and newborn telomere length. Am J Obstet Gynecol. 2016;215(1):94. e1-94.e8. doi:10.1016/j.ajog.2016.01.177
Salihu HM, Pradhan A, King L, et al. Impact of intrauterine tobacco exposure on fetal telomere length. Am J Obstet Gynecol. 2015;212(2):205.e1-205.e8. doi:10.1016/j.ajog.2014.08.026
Theall KP, McKasson S, Mabile E, Dunaway LF, Drury SS. Early hits and long-term consequences: tracking the lasting impact of prenatal smoke exposure on telomere length in children. Am J Public Health. 2013;103(S1):S133-S135. doi:10.2105/AJPH.2012.301208
Mirzakhani H, de Vivo I, Leeder JS, et al. Early pregnancy intrauterine fetal exposure to maternal smoking and impact on fetal telomere length. Eur J Obstet Gynecol Reprod Biol. 2017; 218:27–32. doi:10.1016/j.ejogrb.2017.09.013
Martens DS, Plusquin M, Gyselaers W, de Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016;14(1):148. doi:10.1186/s12916-016-0689-0
Entringer S, Epel ES, Lin J, et al. Maternal folate concentration in early pregnancy and newborn telomere length. Ann Nutr Metab. 2015;66(4):202–208. doi:10.1159/000381925
Bijnens E, Zeegers MP, Gielen M, et al. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int. 2015;79:1–7. doi:10.1016/j.envint.2015. 02.008
Martens DS, Cox B, Janssen BG, et al. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. 2017;171(12):1160. doi:10.1001/jamapediatrics.2017. 3024
Lin S, Huo X, Zhang Q, et al. Short placental telomere was associated with cadmium pollution in an electronic waste recycling town in china. Lustig AJ, ed. PLoS One. 2013;8(4):e60815. doi:10. 1371/journal.pone.0060815
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arias-Sosa, L.A. Understanding the Role of Telomere Dynamics in Normal and Dysfunctional Human Reproduction. Reprod. Sci. 26, 6–17 (2019). https://doi.org/10.1177/1933719118804409
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118804409