Abstract
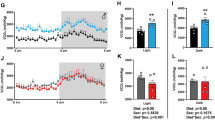
Maternal obesity negatively impacts the placenta, being associated with increased inflammation, decreased mitochondrial respiration, decreased expression of brain-derived neurotrophic factor (BDNF), and its receptor, tropomyosin receptor kinase B (TRKB). TRKB induction by 7,8-dihydroxyflavone (7,8-DHF) improves energy expenditure in an obesity animal model. We hypothesized that TRKB activation would improve mitochondrial respiration in trophoblasts from placentas of obese women. Placentas were collected from lean (pre-pregnancy BMI < 25) and obese (pre-pregnancy BMI > 30) women at term following cesarean section delivery without labor. Cytotrophoblasts were isolated and plated, permitting syncytialization. At 72 hours, syncytiotrophoblasts (STs) were treated for 1 hour with 7,8-DHF (10 nM–10 M), TRKB antagonists (ANA-12 (10 nM–1 M), Cyclotraxin B (1 nM-1M)), or vehicle. Mitochondrial respiration was measured using the XF24 Extracellular Flux Analyzer. TRKB, MAPK, and PGC1α were measured using Western blotting. Maternal obesity was associated with decreased mitochondrial respiration in STs; however, 7,8-DHF increased basal, ATP-coupled, maximal, spare capacity, and nonmitochondrial respiration. A 10 μM dose of 7,8-DHF reduced spare capacity in STs from lean women, with no effect on other respiration parameters. 7,8-DHF had no effect on TRKB phosphorylation; however, there was a concentration-dependent decrease of p38 MAPK phosphorylation and increase of PGC1μ in STs from obese, but not in lean women. TRKB antagonism attenuated ATP-coupled respiration, maximal respiration, and spare capacity in STs from lean and obese women. 7,8-DHF improves mitochondrial respiration in STs from obese women, suggesting that the obese phenotype in the placenta can be rescued by TRKB activation.
Similar content being viewed by others
References
Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291.
Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2): 234–239.
Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8): 2070–2076.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374.
Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197(3): 223–228.
Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F378–F382.
Reece EA. Obesity, diabetes, and links to congenital defects: a review of the evidence and recommendations for intervention. J Matern Fetal Neonatal Med. 2008;21(3):173–180.
Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46.
Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(pt 1):25–30.
Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinolo Metab. 2005;90(7):4299–4308.
Aye IL, Lager S, Ramirez VI, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129.
Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5): E419–E425.
Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes.(Load). 2015;39(8):1274–1281.
Barker DJ. The effect of nutrition of the fetus and neonate on cardiovascular disease in adult life. Proc Nutr Soc. 1992;51(2): 135–144.
Barker DJ. Intrauterine programming of coronary heart disease and stroke. Acta Paediatr Suppl. 1997;423:178–182;discussion 183.
Barker DJ, Gluckman PD, Robinson JS. Conference report: fetal origins of adult disease—report of the First International Study Group, Sydney, 29-30 October 1994. Placenta. 1995;16(3): 317–320.
Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185(1-2):135–144.
Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006;572(pt 1):45–50.
Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2013;34(1):27–46.
Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194.
Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropinreleasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84(6):1997–2001.
Odagiri E, Sherrell BJ, Mount CD, Nicholson WE, Orth DN. Human placental immunoreactive corticotropin, lipotropin, and beta-endorphin: evidence for a common precursor. Proc Nati AcadSci USA. 1979;76(4):2027–2031.
Ramamoorthy S, Leibach FH, Mahesh VB, Ganapathy V. Active transport of dopamine in human placental brush-border membrane vesicles. Am J Physio. 1992;262(5 pt 1):C1189–C1196.
Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343): 347–350.
Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7.
Wu X, Xie C, Zhang Y, Fan Z, Yin Y, Blachier F. Glutamateglutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 2015;47(1):45–53.
Garces MF, Sanchez E, Torres-Sierra AL, et al. Brain-derived neurotrophic factor is expressed in rat and human placenta and its serum levels are similarly regulated throughout pregnancy in both species. Clin Endocrinol (Oxf). 2014;81(1):141–151.
Prince CS, Maloyan A, Myatt L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta. 2017;49:55–63.
Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7(5):695–702.
Yoshida T, Ishikawa M, Niitsu T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7(8):e42676.
Mou Z, Hyde TM, Lipska BK, et al. Human obesity associated with an intronic SNP in the brain-derived neurotrophic factor locus. Cell Rep. 2015;13(6):1073–1080.
Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–123.
Markham A, Cameron I, Bains R, et al. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. EurJNeurosci. 2012;35(3):366–374.
Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20(5):1189–1196.
Fujita K, Tatsumi K, Kondoh E, et al. Differential expression and the anti-apoptotic effect of human placental neurotrophins and their receptors. Placenta. 2011;32(10):737–744.
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401–416.
Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TRKB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22(3):399–408.
Jang SW, Liu X, Yepes M, et al. A selective TRKB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl AcadSci USA. 2010;107(6):2687–2692.
Liu X, Obianyo O, Chan CB, et al. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TRKB receptor. J Biol Chem. 2014;289(40):27571–27584.
Agrawal R, Tyagi E, Vergnes L, Reue K, Gomez-Pinilla F. Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim Biophys Acta. 2014;1842(4):535–546.
Chan CB, Tse MC, Liu X, et al. Activation of muscular TRKB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol. 2015;22(3):355–368.
Bax CM, Ryder TA, Mobberley MA, Tyms AS, Taylor DL, Bloxam DL. Ultrastructural changes and immunocytochemical analysis of human placental trophoblast during short-term culture. Placenta. 1989;10(2):179–194.
Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010;(46):e2511. D0I:10.3791/2511.
Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–823.
Maloyan A, Mele J, Muralimanohara B, Myatt L. Measurement of mitochondrial respiration in trophoblast culture. Placenta. 2012;33(5):456–458.
HanBH, D’Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. NeurobiolDis. 2000;7(1):38–53.
Nakagawa T, Tsuchida A, Itakura Y, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49(3):436–444.
Chen J, Chua KW, Chua CC, et al. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011;499(3):181–185.
Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19(6):1290–1300.
Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TRKB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121(5):1846–1857.
Cazorla M, Jouvenceau A, Rose C, et al. Cyclotraxin-B, the first highly potent and selective TRKB inhibitor, has anxiolytic properties in mice. PloS One. 2010;5(3):e9777.
Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391.
Chen LW, Lin MW, Hsu CM. Different pathways leading to activation of extracellular signal-regulated kinase and p38 MAP kinase by formyl-methionyl-leucyl-phenylalanine or platelet activating factor in human neutrophils. J Biomed Sci. 2005;12(2): 311–319.
Yang JM, Vassil AD, Hait WN. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol. 2001;60(4): 674–680.
McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8): 1263–1284.
Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Molr Cell. 1999;4(4):585–595.
Semple RK, Crowley VC, Sewter CP, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord. 2004;28(1):176–179.
Crunkhorn S, Dearie F, Mantzoros C, et al. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(21):15439–15450.
Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci USA. 2001;98(17):9713–9718.
Fan M, Rhee J, St-Pierre J, et al. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18(3): 278–289.
LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phos-phorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003.
Valle I, Alvarez-Barrientos A, ArzaE, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system invascular endothelial cells. Cardiovasc Res. 2005;66(3):562–573.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prince, C.S., Maloyan, A. & Myatt, L. Tropomyosin Receptor Kinase B Agonist, 7,8-Dihydroxyflavone, Improves Mitochondrial Respiration in Placentas From Obese Women. Reprod. Sci. 25, 452–462 (2018). https://doi.org/10.1177/1933719117716776
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117716776