Abstract
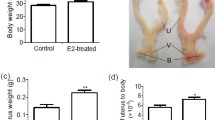
The uterosacral ligaments (USLs) are key support structures of the uterus and upper vagina. Previously, we have shown that HOXA11 is necessary for the development of the USLs, is deficient in women with pelvic organ prolapse (POP) and regulates expression of extracellular matrix (ECM) proteins. Here we sought to determine ifHOXA11 regulates cell proliferation in the USLs in women. Like others, we have found that, there is decreased cellularity in prolapsed USLs compared to USLs in women with normal pelvic support. We have also demonstrated that HOXA11 promotes cell proliferation in murine fibroblasts and primary human USL cells in vitro. These findings support a relationship between HOXA11 expression, rates of proliferation and phenotypic abnormalities in the USL. Based on these findings, we sought to determine if HOXA11 regulates p53, a tumor suppressor gene which controls progression through the cell cycle and regulates ECM genes. We have demonstrated that expression of HOXA11 represses expression of p53, suggesting a mechanism by which HOXA11 regulates of the morphology and integrity of the USLs. A better understanding of the influence of these genes on the homeostasis of the ECM and interactions with each other may prove beneficial in defining the underlying etiologies of the development of POP and aid in the development of new treatment options for women with this disorder.
Similar content being viewed by others
References
Campbell RM. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59(1):1–12.
Cole EE, Leu PB, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2005;97(2):345–348.
Gabriel B, Denschlag D, Gobel H, et al. Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(6):475–479.
DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717–1724.
Samuelsson EC, Victor FT, Tibblin G, Svardsudd K. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299–305.
Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038.
Drutz H, Alarab M. Pelvic organ prolapse: demographics and future growth prospects. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S6-S9.
Gabriel B, Watermann D, Hancke K, et al. Increased expression of metalloproteinase 2 in uterosacral ligaments associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):478–482.
Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG. 2006;113(1):39–46.
Suzme R, Yalcin O, Gurdol F, Gungor F, Bilir A. Connective tissue alterations in women with pelvic organ prolapse and urinary incontinence. Acta Obstetric Gynecol Scand. 2007;86(7):882–888.
Takacs P, Nassiri M, Gualtieri M, Candiotti K, Medina C. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2008 [Epub ahead of print].
Reay Jones NHJ, Healy JC, King LJ, Saini S, Shousha S, Allen-Mersh TG. Pelvic connective tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br J Surg. 2003;90(4):466–472.
Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57(6):1338–1345.
Connell KA, Guess MK, Chen H, Andikyan V, Bercik R, Taylor HS. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J Clin Invest. 2008;118(3):1050–1055.
Kökçü A, Yanik F, Çetinkaya M, Alper T, Kandemir B, Malatyalioglu E. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. 2002;266(2):75–78.
Cole EE, Leu P, Gomelsky A, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2005;97(2):345–348.
Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17.
Jänicke RU, Sohn D, Schulze-Osthoff K. The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ. 2008;15(6):959–976.
Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7): 1379–1384.
Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endo-metrium. J Clin Endocrinol Metab. 1999;84(3):1129–1135.
Chumakov PM. Versatile functions of p53 protein in multicel-lular organisms. Biochemistry (Mosc). 2007;72(13):1399–1421.
Kastan MB, Zhan Q, El-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–597.
Ghosh AK, Bhattacharrya S, Varga J. The tumor suppressor p53 abrogates smad-dependent collagen gene induction in mesenchymal cells. J Biol Chem. 2004;279(46):47455–47463.
Alexandrova A, Ivanov A, Chumakov P, Kopnin B, Vasiliev J. Changes in p53 mouse fibroblasts and extracellular matrix organization. Oncogene. 2000;19(50):5826–5830.
Iotsova V, Stehelin D. Downregulation of fibronectin gene expression by the p53 tumor suppressor protein. Cell Growth Differ. 1996;7(5):629–634.
Bian J, Sun YI. Transcriptional activation by the p53 of the human type IV collagenase (gelatinase A or matrix metallo-proteinase 2) promoter. Mol Cell Biol. 1997;17:6330–6338.
Sun Y, Cheung JM, Martel-Pelletier J, et al. Wild type and mutant p53 differentially regulate the gene expression of human collagenase–3 (hMMP-13). J Biol Chem. 2000;275(15): 11327–11332.
Ala-aho R, Grenman R, Seth P, Kahari V-M. Adenoviral delivery of p53 gene suppresses expression of collagenase–3 (MMP13) in squamous carcinoma cells. Oncogene. 2002;21:1187–1195.
Bai SW, Chung DJ, Yoon JM, Shin JS, Kim SK, Park KH. Roles of estrogen receptor, progesterone receptor, p53 and p21in pathogenesis of pelvic organ prolapse. Int Urogynecol J. 2005;16:492–496.
Yamamoto M, Aoyagi M, Akazawa K, Tajima S, Yamamoto K. Decrease in p53 protein in cultured cardinal ligament fibroblasts from patients with prolapsus uteri. Cell Bio Int. 1998;22(1):31–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connell, K.A., Guess, M.K., Chen, H.W. et al. HOXA11 Promotes Fibroblast Proliferation and Regulates p53 in Uterosacral Ligaments. Reprod. Sci. 16, 694–700 (2009). https://doi.org/10.1177/1933719109334260
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719109334260