Abstract
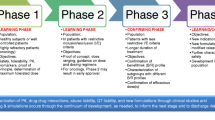
There are many decision points along the product development continuum. Formal clinical milestones, such as the end of phase 1, phase 2a (proof of mechanism or proof of concept), and phase 2b provide useful decision points to critically evaluate the accumulating data. At each milestone, sound decisions begin with asking the right questions and choosing the appropriate design as well as criteria to make go/no-go decisions. It is also important that knowledge about the new investigational product, gained either directly from completed trials or indirectly from similar products for the same disorder, be systematically incorporated into the evaluation process. In this article, we look at metrics that go beyond type I and type II error rates associated with the traditional hypothesis test approach. We draw on the analogy between diagnostic tests and hypothesis tests to highlight the need for confirmation and the value of formally updating our prior belief about a compound’s effect with new data. Furthermore, we show how incorporating probability distributions that characterize current evidence about the true treatment effect could help us make decisions that specifically address the need at each clinical milestone. We illustrate the above with examples.
Similar content being viewed by others
References
Masia N. The cost of developing a new drug. In Focus on Intellectual Property Rights. US Department of State; April 23, 2008. http://www.america.gov/st/business-English/2008/April/20080429230904myleen0.5233981.html.
Parmar MK, Ungerleider RS, Simon R. Assessing whether to perform a confirmatory randomized clinical trial. J Natl Cancer Inst. 1996;88:1645–1651.
Lee SJ, Zelen M. Clinical trials and sample size considerations: another perspective. Stat Sci. 2000;15(2):95–110.
O’Hagan A, Stevens JW, Campbell MJ. Assurance in clinical trial design. Pharm Stat. 2005;4:187–201.
Chuang-Stein C. Sample size and the probability of a successful trial. Pharm Slat. 2006;5:305–309.
Hobbs BP, Carlin BP. Practical Bayesian design and analysis for drug and device clinical trials. J Biopharm Stat. 2008;18:54–80.
Kowalski KG, Ewy W, Hutmacher MM, Miller R, Krishnaswami S. Model-based drug development—a new paradigm for efficient drug development. Biopharm Rep. 2007;15(2):2–22.
Kowalski KG, French JL, Smith MK, Hutmacher MM. A model-based framework for quantitative decision-making in drug development. Presented at the American Conference on Pharmacometrics. Tucson AZ, March 12, 2008. http://tucson2008.go-acop.org/pdfs/8-Kowalski_FINAL.pdf
Kowalski KG, Olson S, Remmers AE, Hutmacher MM. Modeling and simulation to support dose selection and clinical development of SC-75416. a selective COX-2 inhibitor for the treatment of acute and chronic pain. Clin Pharm Ther. 2008;83:857–866.
Smith MK, French J, Kowalski K, Ewy W. Enhanced quantitative decision making—reducing the likelihood of incorrect decisions. Presented at the PAGE (Population Approach Group in Europe) Predictive Modeling in Drug Development Satellite Meeting, St. Petersburg, Russia. June 23, 2009.
Chuang-Stein C, Yang R. A revisit of sample size decision in confirmatory trials. Stat Biopharm Res. 2010;2:239–248.
Fleiss JL. Design and Analysis of Clinical Experiments. New York: Wiley: 1999.
Browner WS, Newman TB. Are all significant p-values created equal? JAMA. 1987;257:2459–2463.
Pater JL, Willan AR. Clinical trials as diagnostic tests. Control Clin Trials. 1984;5:107–113.
Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–442.
US Department of Health and Human Services. Food and Drug Administration. Innovation or stagnation? Challenge and opportunity on the critical path to new medical products. 2004. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm113411.pdf.
Peck CC, Barr WH, Benet LZ, et al. Opportunities for integration of pharmacokinetics, pharmacodynamics and toxicokinetics in rational drug development. Clin Pharmacol Ther. 1992;51:465–473.
Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharmacol Ther. 1997;61:275–291.
Grasela TH, Dement CW, Kolerman OG, et al. Pharmacometrics and the transition to model-based development. Clin Pharm Ther. 2007;82:137–142.
Lalonde RL, Kowalski KG, Hutmacher MM, et al. Model-based drug development. Clin Pharm Ther. 2007;82:21–32.
Zhang L, Sinha V, Forgue T, et al. Model-based drug development: the road to quantitative pharmacology. J Pharmacokinet Pharmacodyn. 2006;33:369–393.
Zhang L, Pfister M, Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. AAPS J. 2008;10:552–559.
Ades A, Sutton AJ. Multiple parameter evidence synthesis in epidemiology and medical decisionmaking: current approaches. J R Stat Soc. A. 2006;169:5–35.
Mandema JW, Hermann D, Wang W, et al. Model-based development of gemcabene, a new lipid-altering agent. AAPS J. 2005;7:E513–E522.
Ahn JE, French JL. Longitudinal aggregate data model-based meta-analysis with NONMEM: approaches to handling within treatment arm correlation. J Pharmacokinet Pharmacodyn. 2010;37:179–201.
Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T. Alzheimer’s Disease Working Group. A disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement. 2010;6:39–53.
Smith MK, Marshall S. A Bayesian design and analysis for dose-response using informative prior information. J Biopharm Stat. 2006;16:695–709.
Santen G, van Zwet E, Danhof M, Della Pasqual O. From trial and error to trial simulation. Part 1: the importance of model-based drug development for antidepressant drugs. Clin Pharmacol Ther. 2009;86:248–254.
Santen G, Horrigan J, Danhof M, Delia Pasqual O. From trial and error to trial simulation. Part 2: an appraisal of current beliefs in the design and analysis of clinical trials for antidepressant drugs. Clin Pharmacol Ther. 2009;86:255–261.
Sultana SR, Marshall S, Davis J, Littman BH. Experiences with dose finding in patients in early drug development: the use of biomarkers in early decision making in appropriate dose selection—how to optimize clinical drug development. Ernst Schering Foundation Symp Proc. 2007;59:65–79.
de Greef R. Target occupancy biomarkers: schizophrenia. In: Danhof M, Van der Graaf PH, Holford NHG, eds. Measurement and Kinetics of in Vivo Drug Effects: Advances in Simultaneous Pharmacokinetic/Pharmacodynamic Modelling. 6th International Symposium. Leiden/Amsterdam Center for Drug Research: 2010:67–70.
Danhof M, Della Pasqua O, Knibbe CAJ, de Lange ECM, Voskuyl RA, Ploeger BA. Quantitative systems pharmacology: what are the targets? In: Danhof M, Van der Graaf PH, Holford NHG, eds. Measurement and Kinetics of in Vivo Drug Effects: Advances in Simultaneous Pharmacokinetic/Pharmacodynamic Modelling. 6th International Symposium. Leiden/Amsterdam Center for Drug Research: 2010:5–12.
Van der Graaf PH, Benson N. Bridging systems biology and PKPD: towards novel drugs. In: Danhof M, Van der Graaf PH, Holford NHG, eds. Measurement and Kinetics of in Vivo Drug Effects: Advances in Simultaneous Pharmacokinetic/Pharmacodynamic Modelling. 6th International Symposium. Leiden/Amsterdam Center for Drug Research: 2010:17–22.
Chuang-Stein C, Kirby S, Hirsch I, Atkinson G. The role of the minimum clinically important difference and its impact on designing a trial. Pharm Stat. 2010. Published online. DOI 10.1002/pst459.
Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomized phase II study. Lancet. 2008;371:2101–2108.
Wang SJ, Hung HMJ, O’Neill RT. Adapting the sample size planning of a phase III trial based on phase II data. Pharm Stat. 2006;5:85–97.
Carroll KJ. Back to basics: explaining sample size in outcome trials, are statisticians doing a thorough job? Pharm Stat. 2009;8:333–345.
Staquet MJ, Rozencweig M, Von Hoff DD, Mugia FM. The delta and epsilon errors in the assessment of clinical trials. Cancer Treat Rep. 1979;63:1917–1921.
Simon R. Randomized clinical trials and research strategy. Cancer Treat Rep. 1982;66:1083–1087.
Simon R. Some practical aspects of the interim monitoring of clinical trials. Stat Med. 1994;13:1401–1409.
Berger J, Sellke T. Testing of a point null hypothesis: the irreconcilability of significance levels and evidence. J Am Stat Assoc. 1987;82:112–122.
Liu PY, LeBlanc M, Desai M. False positive rates of randomized phase II designs. Control Clin Trials. 1999;20:343–352.
Stallard N, Todd S, Whitehead J. Estimation following selection of the largest of two normal means. J Stat Plan Infer. 2008;138:1629–1638.
Bauer P, Koenig F, Brannath W, Posch M. Selection and bias—two hostile brothers. Stat Med. 2010;29(1):1–13.
Julious SA, Swank DJ. Moving statistics beyond the individual clinical trial: applying decision science to optimize a clinical development plan. Pharm Stat. 2005;4(1):37–46.
Burman CF, Grieve AP, Senn S. Decision analysis in drug development. In: Dmitrienko A, Chuang-Stein C, Agostino R, ed. Pharmaceutical Statistics Using SAS: A Practical Guide. Cary, NC: SAS Institute; 2007:385–428.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chuang-Stein, C., Kirby, S., French, J. et al. A Quantitative Approach for Making Go/No-Go Decisions in Drug Development. Ther Innov Regul Sci 45, 187–202 (2011). https://doi.org/10.1177/009286151104500213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286151104500213