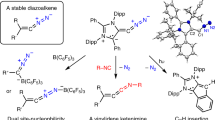
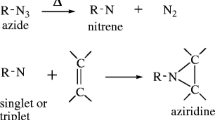
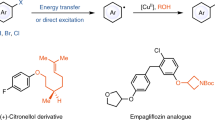
Abstract
Since alkynes have higher symmetry than olefins, it is not easy to infer the mechanism of a triplet carbene’s addition to an alkyne by traditional product analysis studies. Specifically, no stereochemical information which might offer insight into the carbene’s spin state can be extracted from the cyclopropene products. In 1971, Hendrick, Baron, and Jones showed that diphenylcarbene reacts with terminal alkynes in solution to produce indenes via a “self-trapping” vinylcarbene. They also examined the diphenylcarbene reaction with disubstituted alkynes and found at most trace amounts of the “self-trapping” indene product.
In this work, we report the direct observation by organic matrix EPR of the vinylcarbenes generated from triplet fluorenylidene and terminal alkynes. Our efforts to confirm the identities of these intermediates by independent synthesis, intermolecular trapping, and an intramolecular “self-trapping” method-halogen-migration-are also recounted. These findings are among the few instances in which fluorenylidene undergoes carbon-carbon bond formation rather than atom abstraction reactions in a low-temperature matrix, and in which the biradical adduct of a triplet carbene and a π-bonded substrate can be directly observed.
Similar content being viewed by others
References and notes
W.J. Baron, M.E. Hendrick, and M. Jones, Jr, J. Am. Chem. Soc. 95, 6286 (1973).
W.J. Baron, M.R. DeCamp, M.E. Hendrick, M. Jones, Jr., R.H. Levin, and M.B. Sohn. In: Carbenes, M. Jones, Jr. and R.A. Moss, (Eds.). vol. I, pp. 73–84. Wiley, New York (1973).
D. Griller, A.S. Nazran, and J.C. Scaiano, Acc. Chem. Res. 17, 283 (1984).
M.S. Platz. In: Kinetics and Spectroscopy of Carbenes and Biradicals, M.S. Platz (Ed.). pp. 143. Plenum, New York (1990).
T.G. Savino, V.P. Senthilnathan, and M.S. Platz, Tetrahedron, 42, 2167 (1986).
B.B. Wright and M.S. Platz, J. Am. Chem. Soc. 106, 4175 (1984).
N.J. Turro, Tetrahedron, 38, 809 (1982).
P.B. Grasse, B.-E. Brauer, J.J. Zupancic, K.J. Kaufmann, and G.B. Schuster, J. Am. Chem. Soc. 105, 6833 (1983).
R.A. Moss and M.A. Joyce, J. Am. Chem. Soc. 100, 4475 (1978).
a) H. Tomioka, S. Suzuki, Y. Izawa, J. Am. Chem. Soc., 104, 3156 (1982); b) J. Ruzicka, E. Leyva, and M.S. Platz, J. Am. Chem. Soc. 114, 897 (1992).
For a striking case of matrix-locked ESR behavior in a diaryl carbene, see A.S. Nazran, F.L. Lee, E.J. Gabe, Y. Lepage, D.J. Northcott, J.M. Park, and D. Griller, J. Phys. Chem. 88, 5251 (1984) and references therein.
We have obtained the crystal structure for compound 13a. It is essentially identical to the MNDO optimized structure. Unfortunately, we were unable to crystallize the 3,3-diphenyl-5-phenyl- 3H-pyrazole, so in order to make a uniform comparison, the geometries shown in Figure 4 were both optimized using the MNDO method.
J.E. Jackson and T. O’Brien, Jr, J. Phys. Chem. 92, 2686 (1988).
E. Leyva, R.L. Barcus, and M.S. Platz, J. Am. Chem. Soc. 108, 7786 (1986).
P.P. Gaspar, C.-T. Lin, W. Dunbar, D.P. Mack, and P. Balasubramanian, J. Am. Chem. Soc. 106, 2128 (1984).
R.A. Moss and U.-H. Dolling, J. Am. Chem. Soc. 93, 954 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, M.S., Jackson, J.E. Low temperature carbene-to-carbene homologations. Res. Chem. Intermed. 20, 223–247 (1994). https://doi.org/10.1163/156856794X00216
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856794X00216