Abstract
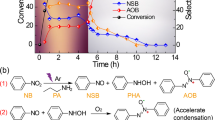
A easy and environmentally friendly method for the photochemical functionalisation of the heterocyclic bases is described. The selective introduction of the amido group of formamide, using sunlight and air in the presence of TiO2, has allowed to obtain the desired products in mild conditions with high conversions. The comparison of these results with those previously reported, in the presence of H2O2 instead of air, clarifies some aspects of the reaction mechanism.
Similar content being viewed by others
References
A. Studer and M. Bossart, in Radicals in Organic Synthesis, Vol. II, P. Renauld and M. P. Sibi (Eds), p. 62, Wiley-VCH, New York, NY (2001).
F. Minisci, Synthesis, 1 (1973).
F. Minisci, Top. Curr. Chem. 62, 1 (1976).
F. Minisci, E. Vismara and F. Fontana, Heterocycles 28, 489 (1989).
F. Minisci, F. Fontana and E. Vismara, J. Heterocycl. Chem. 27, 79 (1990).
O. Porta and F. Minisci, in Handbook of C−H Transformations Gerard Dyker (Ed.), p. 212. Wiley-VCH, New York, NY (2005).
M. A. Fox and M. T. Dulay, Chem. Rev. 93, 341 (1993).
A. Maldotti, A. Molinari and R. Amadelli, Chem. Rev. 102, 3811 (2002).
T. Del Giacco, M. Ranchella, C. Rol and G. V. Sebastiani, J. Phys. Org. Chem. 13, 745 (2000).
M. Ranchella, C. Rol and G. V. Sebastiani, J. Chem. Res. (Suppl.), 239 (2002).
T. Del Giacco, C. Rol and G. V. Sebastiani, J. Phys. Org. Chem. 16, 127 (2003).
H. Keck, W. Schindler, F. Knock and H. Kisch, Chem. Eur. J. 3, 1638 (1997).
H. Kisch, Adv. Photochem. 62, 93 (2001).
T. Caronna, C. Gambarotti, L. Palmisano, C. Punta and F. Recupero, Chem. Commun., 2350 (2003).
T. Caronna, C. Gambarotti, L. Palmisano, C. Punta and F. Recupero, J. Photochem. Photobiol. A: Chem. 171, 237 (2005).
M. Schiavello, Heterogeneous Photocatalysis, Wiley, New York, NY (1995).
F. Minisci, E. Vismara, F. Fontana, G. Morini, M. Serravalle and C. Giordano, J. Org. Chem. 52, 730 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caronna, T., Gambarotti, C., Mele, A. et al. A green approach to the amidation of heterocyclic bases: the use of sunlight and air. Res. Chem. Intermed. 33, 311–317 (2007). https://doi.org/10.1163/156856707779238603
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856707779238603