Abstract
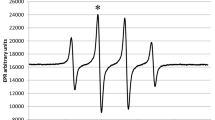
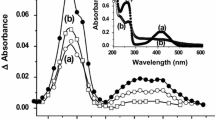
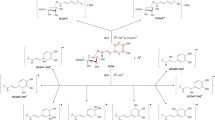
At near neutral pH (approx. 5.5), the OH-adduct of chlorogenic acid (CGA), formed on pulse radiolysis of N2O-saturated aqueous CGA solutions (λ max = 400 and 450 nm) with k = 9 × 109 dm3 mol−1 s−1, rapidly eliminates water (k = 1 × 103 s−1) to give a resonance-stabilized phenoxyl type of radical. Oxygen rapidly adds to the OH-adduct of CGA (pH 5.5) to form a peroxyl type of radical (k = 6 × 107 dm3 mol−1 s−1). At pH 10.5, where both the hydroxyl groups of CGA are deprotonated, the rate of reaction of · OH radicals with CGA was essentially the same as at pH 5.5, although there was a marked shift in the absorption maximum to approx. 500 nm. The CGA phenoxyl radical formed with more specific one-electron oxidants, viz., Br ·−2 and N ·3 radicals show an absorption maximum at 385 and 500 nm, k ranging from 1–5.5 × 109 dm3 mol−1 s−1. Reactions of other one-electron oxidants, viz., NO ·2 , NO· and CCl3OO· radicals, are also discussed. Repair rates of thymidine, cytidine and guanosine radicals generated pulse radiolytically at pH 9.5 by CGA are in the range of (0.7–3) × 109 dm3 mol−1 s−1.
Similar content being viewed by others
References
T. Walle, Free Radic. Biol. Med. 36, 829 (2004).
T. Cornwell, W. Cohick and I. Rask, Phytochemistry 65, 995 (2004).
J. F. Weiss and M. R. Landauer, Toxicology 189, 1 (2003).
K. Robards J. Chromatogr. A 1000, 657 (2003).
G. Loo, J. Nutrit. Biochem. 14, 64 (2003).
L. Le Marchand, Biomed. Pharmacother. 56, 296 (2002).
F. Visioli, L. Borsani and C. Galli, Cardiovasc. Res. 47, 419 (2000).
I. B. Afanas’ev, A. I. Dorozhko, A. V. Brodskii, V. A. Kostyuk and A. I. Potapovitch, Biochem. Pharmacol. 38, 1763 (1989).
H. S. Mahal, Transient characteristics of some corrosion inhibitor compounds and phenolic antioxidants as studied by pulse radiolysis, Ph.D. thesis, Bhabha Atomic Research Center, Mumbai (1996).
G. K. Sharma, A. D. Semwal, M. C. N. Murthy and S. S. Arya, Food Chem. 60, 19 (1997).
C. X. Zhang, H. Wu and C. Weng, Food Chem. 84, 219 (2004).
L. Bravo, Nutrit. Rev. 56, 317 (1998).
C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radic. Biol. Med. 20, 933 (1996).
Y. Hanaski, S. Ogawa and S. Fukui, Free Radic. Biol. Med. 16, 845 (1994).
H. Yamaski and S. C. Grace, FEBS Lett. 422, 377 (1998).
C. Castelluccio, G. Paganga, N. Melikian, G. P. Bolwell, J. Pridham, J. Sampson and C. Rice-Evans, FEBS Lett. 368, 188 (1995).
F.-A. Chen, A.-B. Wu and C.-Y. Chen, Food Chem. 86, 479 (2004).
M. Ohnishi, H. Morishita, H. Iwahashi, S. Toda, Y. Shirataki, M. Kimura and R. Kido, Phytochem. 36, 579 (1994).
E. Pelle, D. Maes, G. A. Padulo, K. E. Kim and W. P. Smith, Arch. Biochem. Biophys. 283, 234 (1990).
T. Mukherjee, in: Atomic Molecular and Cluster Physics, S. A. Ahmad (Ed.), p. 299. Narosa, New Delhi (1997).
E. M. Fielden, in: The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis, J. H. Baxandale and F. Busi (Eds), p. 49. Reidel, Dordrecht (1982).
H. S. Mahal, H. S. Sharma and T. Mukherjee, Free Radic. Biol. Med. 26, 557 (1999).
E. Mvula, M. N. Schuchmann and C. von Sonntag, J. Chem. Soc. Perkin Trans. 2, 264 (2001).
D. Wang, I. György, K. Hildenbrant and C. von Sonntag, J. Chem. Soc. Perkin Trans. 2, 45 (1994).
K. Bobrowski, J. Chem. Soc. Faraday Trans. 80, 1377 (1984).
H. Pal and T. Mukherjee, J. Ind. Chem. Soc. 70, 409 (1993).
S. Solar, W. Solar, N. Getoff, J. Holcman and K. Sehested, J. Chem. Soc. Faraday Trans. 78, 2467 (1982).
S. V. Jovanovic, Y. Hara, S. Steenken and M. G. Simic, J. Am. Chem. Soc. 117, 9881 (1995).
P. Wardman, J. Phys, Chem. Ref. Data 18, 1637 (1989).
Z. B. Alfassi and R. H. Schuler, J. Phys. Chem. 89, 3359 (1985).
G. G. Duthie and K. J. Wahle, Biochem. Soc. Trans. 18, 1051 (1990).
A. J. Frank and M. Gratzel, Inorg. Chem. 21, 3834 (1982).
W. A. Pryor and J. W. Lightsey, Science 214, 435 (1981).
S. Moncada, R. M. J. Palmer and E. A. Higgs, Pharmacol. Rev. 43, 109 (1991).
H. S. Mahal, L. P. Badheka and T. Mukherjee, Res. Chem. Intermed. 27, 595 (2001).
O. I. Arouma, J. P. E. Spencer, J. Butler and B. Halliwell, Free Radic. Res. 22, 187 (1995).
J. Mönig, M. Göbl and K.-D. Asmus, J. Chem. Soc. Perkin Trans. 2, 647 (1985).
W. R. Sousa, C. da Rocha, C. L. Cardoso, D. H. L. Silva and M. V. B. Zanoni, J. Food Composit. Anal. 17, 619 (2004).
M. M. Baizer and H. Lund, Organic Electrochemistry. Harper and Row, New York, NY (1972).
H. Kasai, S. Fukada, Z. Yamaizumi, S. Sugie and H. Mori, Food Chem. Toxicol. 38, 467 (2000).
M. G. Simic and S. V. Jovanovic, J. Am. Chem. Soc. 111, 5778 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahal, H.S., Mukherjee, T. Radical scavenging reactions of chlorogenic acid: A pulse radiolysis study. Res Chem Intermed 32, 671–682 (2006). https://doi.org/10.1163/156856706778400343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856706778400343