Abstract
Graves' disease is a thyroid-specific autoimmune disorder in which the body makes antibodies to the thyroid-stimulating hormone receptor leading to hyperthyroidism. Therapeutic options for the treatment of Graves' disease include medication, radioactive iodine ablation, and surgery. Radioactive iodine is absolutely contraindicated in pregnancy as exposure to I-131 to the fetal thyroid can result in fetal hypothyroidism and cretinism. Here we describe a case of a female patient with recurrent Graves' disease, who inadvertently received I-131 therapy when she was estimated to be eight days pregnant. This was despite the obtaining of a negative history of pregnancy and a negative urine pregnancy test less than 24 hours prior to ablation. At birth, the infant was found to have neonatal Graves' disease. The neonatal Graves' disease resolved spontaneously. It was suspected that the fetal thyroid did not trap any I-131 as it does not concentrate iodine until 10 weeks of gestation.
Similar content being viewed by others
1. Introduction
Radioactive iodine thyroid ablation has been shown to be an inexpensive, safe, and effective treatment for hyperthyroidism [1]. It is the most commonly used method for treating adult patients with Graves' disease [2]. It is very important not to treat a woman who is pregnant or nursing. Special precautions must be used in women of child-bearing age because of the possible detrimental side effects of fetal exposure [3].
The American College of Radiology (ACR) practice guideline for the performance of therapy with unsealed radiopharmaceutical sources states that pregnancy should be ruled out using one of the following four criteria: (1) a negative hCG test obtained within 72 hours prior to administration of the radiopharmaceutical, (2) documented history of hysterectomy, (3) a postmenopausal state with absence of menstrual bleeding for two years, and (4) premenarche in a child age of 10 years or younger [4]. The Society of Nuclear Medicine (SNM) procedure guideline for therapy of thyroid disease with I-131 states that females of child-bearing age should routinely be tested for pregnancy within 72 hours or less before I-131 treatment. When the patient's history clearly indicates that pregnancy is impossible, the treating physician may omit the pregnancy test [5].
2. Case Report
TJ (not the patient's initials) is an adult female diagnosed with Graves' disease, which was treated with I-131 therapy in December 2004. She had been doing well until June 2006 when she presented to the pediatric endocrinology clinic with reoccurrence of her disease. Her thyroid function tests showed TSH < 0.03 (normal 0.32–5.0 munit/mL), free T4 2.80 (normal 0.71–1.85 ng/dL), and T4 19.1 (normal 5.0–12.0 mcg/dL). Unfortunately, her menstrual history was not documented during this clinic visit. TJ was scheduled for a radioactive iodine uptake and scan with subsequent ablation with I-131. A qualitative urine pregnancy test was performed 24 hours prior to ablation and was negative. A radioiodine uptake and scan revealed a 24-hour uptake of 100% (normal 10%–30%). Informed consent for I-131 therapy was obtained. TJ verified that she was not pregnant and was given an activity of 19.8 mCi of I-131. This dosage was used due to the patient's prior failed therapy.
Approximately four months later, after slipping and falling at work, TJ presented to her physician with a complaint of low back pain. Magnetic resonance imaging (MRI) of her lumbar spine revealed an intrauterine pregnancy. TJ again denied any possibility of being pregnant prior to the MRI. A subsequent ultrasound showed the fetus at approximately 17 4/7-week gestation. It was estimated that the fetus was eight to ten days old at the time of I-131 treatment.
TJ delivered a full-term male infant. Neonatal thyroid function tests revealed suppressed TSH (<0.02, normal 0.32–5.0 munit/mL), elevated T4 (24.4, normal 5.0–12.0 mcg/dL), and free T4 (4.54, normal 0.58–1.64 ng/dL). In addition, the infant had an elevated thyroid receptor antibody (TRab) titer of 82% (negative <10%, intermediate 10%–15%, positive >15%). Based on the laboratory evaluation, it was suspected that the infant had neonatal hyperthyroidism secondary to maternal antibodies. Because he was asymptomatic, a decision was made not to treat but to monitor him closely. Over the next six months, the infant grew, gained weight, and reached developmental milestones appropriately. His thyroid function tests normalized with TSH 0.73 munit/mL, T4 10.3 mcg/dL, free T4 1.11 ng/dL, and TRab <15%.
3. Discussion
Thyroid embryogenesis is largely completed by 10–12-week gestation. At 10-week gestation, the thyroid gland is able to trap and concentrate iodine and synthesize thyroid hormones thyroxine and triiodothyronine [6]. Iodine, including radioactive isotopes, is readily transferred across the placenta [7]. After 10-week gestation, significant exposure to the fetal thyroid can occur from therapeutic doses to the mother, resulting in hypothyroidism and cretinism [8]. There have been no reports of birth defects or childhood malignancy in children born to the mothers who received radioactive iodine for Graves' disease before the 10th week of pregnancy [9]. We believe the infant's thyroid gland was not affected by the I-131 therapy because he was exposed prior to 10-week gestation.
Quantifying the exact absorbed dose by the embryo in this case is difficult and depends on many variables. Using the methodology provided by Russell et al., a rough estimate of the dose that the embryo received can be calculated [10]. This calculation likely overstates the actual absorbed dose, as the model assumes that I-131 crosses the placenta. In this case, due to the placenta being in the early stages of development during the second week of gestation, the amount of I-131 that crossed would be limited. It is estimated that the embryo absorbed dose with I-131 treatment in early pregnancy is 0.072 mGy/MBq [10]. Our patient received a dose of 19.8 mCi (732.6 MBq), which corresponds to an absorbed dose of approximately 5.3 rads after converting from Gy to rads. Again, this likely represents a greater dose than the embryo actually received.
Radiation-induced effects can be broken down into deterministic effects and stochastic effects. Deterministic effects are those that are known to occur at a given radiation threshold. For example, if a patient were to receive an acute dose of 2 Gy to the lens of the eye, the patient will develop a cataract. Stochastic effects are those that can theoretically occur at any radiation dose. For example, any radiation dose could potentially induce a cancer in the future. The probability of a stochastic effect increases as dose increases, but there is no radiation threshold dose below which one can say a stochastic effect will not occur. Therefore, stochastic effects can theoretically occur with any radiation dose. The only known deterministic effect of radiation at an absorbed dose of less than 10 rads is possible spontaneous abortion at 1–14 days postconception. Based on the data above, this dose level (10 rads) was not reached in this patient. On the other hand, stochastic effects are theoretically possible at any level of radiation dose [11]. Thus, the radiation dose to any developing embryo should be minimized, and it is reasonable to ascertain an accurate pregnancy test prior to the use of I-131 therapy.
Studies have demonstrated that the risk of congenital effects have been negligible at doses of 5 rads or less when compared to other risks of pregnancy. In addition, the risk of malformation only significantly increases at doses above 15 rads. Mental retardation has been shown to occur in the offspring of pregnant mothers; however, it is at doses greater than 20 rads and generally after the eighth week of gestation [12]. A screening study of thyroid cancer among individuals exposed to in utero I-131 from the Chernobyl fallout, however, did demonstrate an increased risk of thyroid carcinoma approximately 20 years after the accident [13]. Thus, our estimation that the infant was exposed to approximately 5 rads supports that no deterministic effects should have occurred, but one cannot exclude the future potential for stochastic effects.
As previously stated, the ACR practice guideline for the performance of therapy with unsealed radiopharmaceutical sources states that pregnancy should be ruled out using one of the following four criteria: (1) a negative hCG test obtained within 72 hours prior to administration of the radiopharmaceutical, (2) documented history of hysterectomy, (3) a postmenopausal state with absence of menstrual bleeding for two years, and (4) premenarche in a child age 10 or younger [4]. The SNM procedure guideline for therapy of thyroid disease with I-131 states that females of child-bearing age should routinely be tested for pregnancy within 72 hours or less before I-131 treatment. When the patient's history clearly indicates that pregnancy is impossible, the treating physician may omit the pregnancy test [5]. Neither of the above guidelines specifies whether the pregnancy tests should be quantitative or qualitative.
Typically, serum pregnancy tests are more sensitive than standard urine pregnancy tests because the serum concentration of hCG is significantly higher than its urine concentration [14]. Detection of hCG in maternal serum is evident only after implantation and vascular communication has been established with the decidua by the syncytiotrophoblast, which occurs 8–10 days following conception. Serum hCG can be detected in about 5% of patients eight days after conception and about 98% of patients by 11 days postconception [15]. In contrast, urine pregnancy tests will be positive 15–17 days after conception in 98% of patients [14, 16]. Alternatively, many experts advocate that use of the Ten Day Rule. This rule suggests that radioactive iodine therapy only be administered during the 10 days after the onset of the menstrual period [17]. However, this protocol may not be effective if the patient has irregular menstrual cycles.
One possible suggestion is to incorporate the Ten Day Rule protocol into the current guidelines for patients who have regular 28-day menstrual cycles. For patients with irregular cycles or who require immediate ablation, quantitative serum hCG pregnancy tests on the day of the treatment should be considered. Because serum hCG is detected in 98% of pregnant patients by day 11, recommending the patients to abstain from sexual activity for at least two weeks prior to I-131 therapy may be suggested. This abstinence would cover the gap from the time of conception to the time the serum hCG test becoming positive. However, the physician would have to rely on the patient for providing an accurate menstrual cycle and sexual history.
4. Conclusion
It is imperative to rule out pregnancy prior to the administration of radioactive iodine therapy due to the potential detrimental side effects of fetal exposure. The dose of I-131 in this particular case would be unlikely to result in any deterministic events. However, under the premise that any unnecessary exposure to radiation is important in preventing any potential stochastic events, an accurate pregnancy screening protocol may be warranted in preventing inadvertent I-131 treatment in early pregnancies of women with Graves' disease. The current ACR and SNM guidelines only mandate a hCG test being obtained within 72 hours prior to the treatment. This approach may miss a small number of pregnancies as a serum or urine pregnancy test does not become positive until implantation occurs, which happens 8–10 days postconception. Though we recommend re-evaluating the ACR and SNM guidelines for future clarifications, it is possible that despite combining the patient's history, the Ten Day Rule and the hCG screening, an unsuspected pregnancy may be missed.
References
Iagaru A, McDougall IR: Treatment of thyrotoxicosis. Journal of Nuclear Medicine. 2007, 48 (3): 379-389.
Berg GEB, Michanek AMK, Holmberg ECV, Fink M: Iodine-131 treatment of hyperthyroidism: significance of effective half-life measurements. Journal of Nuclear Medicine. 1996, 37 (2): 228-232.
Stoffer SS, Hamburger JI: Inadvertent
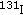 therapy for hyperthyroidism in the first trimester of pregnancy. Journal of Nuclear Medicine. 1976, 17 (2): 146-149.
therapy for hyperthyroidism in the first trimester of pregnancy. Journal of Nuclear Medicine. 1976, 17 (2): 146-149.ACR : Practice Guideline for the Performance of Therapy with Unsealed Radiopharmaceutical Sources. 2005, http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/ro/unsealed_radiopharmaceuticals.aspx
Society of Nuclear Medicine Procedure Guideline for Therapy of Thyroid Disease with Iodine-131 (Sodium Iodine). Version 2.0, http://interactive.snm.org/docs/Therapy%20of%20Thyroid%20Disease%20with%20Iodine-131%20v2.0.pdf
Berg G, Jacobsson L, Nyström E, Gleisner KS, Tennvall J: Consequences of inadvertent radioiodine treatment of Graves' disease and thyroid cancer in undiagnosed pregnancy. Can we rely on routine pregnancy testing?. Acta Oncologica. 2008, 47 (1): 145-149. 10.1080/02841860701558807.
Agency for Toxic Substances & Disease Registry : Radiation exposure from Iodine 131 who is at risk. November 2002, http://www.atsdr.cdc.gov/csem/iodine/whosat_risk.html
Ziessman H, O'Malley J, Thrall J: Endocrine system. The Requisites: Nuclear Medicine. 2006, 76-3
Evans PMS, Webster J, Evans WD, Bevan JS, Scanlon MF: Radioiodine treatment in unsuspected pregnancy. Clinical Endocrinology. 1998, 48 (3): 281-283. 10.1046/j.1365-2265.1998.00423.x.
Russell JR, Stabin MG, Sparks RB, Watson E: Radiation absorbed dose to the embryo/fetus from radiopharmaceuticals. Health Physics. 1997, 73 (5): 756-769. 10.1097/00004032-199711000-00003.
ACR : Practice Guideline for Imaging Pregnant or Potentially Pregnant Adolescents and Women with Ionizing Radiation. 2008, http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/dx/Pregnancy.aspx
Stabin MG, Watson EE, Marcus CS, Salk RD: Radiation dosimetry for the adult female and fetus from iodine-131 administration in hyperthyroidism. Journal of Nuclear Medicine. 1991, 32 (5): 808-813.
Hatch M, Brenner A, Bogdanova T: A screening study of thyroid cancer and other thyroid diseases among individuals exposed in utero to lodine-131 from Chernobyl fallout. Journal of Clinical Endocrinology and Metabolism. 2009, 94 (3): 899-906. 10.1210/jc.2008-2049.
Bohuslavizki KH, Kröger S, Klutmann S, Geiss-Tönshoff M, Clausen M: Pregnancy testing before high-dose radioiodine treatment: a case report. Journal of Nuclear Medicine Technology. 1999, 27 (3): 220-221.
Shields A: Pregnancy diagnosis. April 2009, http://emedicine.medscape.com/article/262591-overview
Chard T: Pregnancy tests: a review. Human Reproduction. 1992, 7 (5): 701-710.
Watson WS: Radioiodine therapy and pregnancy. Journal of Nuclear Medicine. 2005, 46 (8): 1408-1409.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tran, P., DeSimone, S., Barrett, M. et al. I-131 Treatment of Graves' Disease in an Unsuspected First Trimester Pregnancy; the Potential for Adverse Effects on the Fetus and a Review of the Current Guidelines for Pregnancy Screening. Int J Pediatr Endocrinol 2010, 858359 (2010). https://doi.org/10.1155/2010/858359
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1155/2010/858359





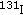 therapy for hyperthyroidism in the first trimester of pregnancy. Journal of Nuclear Medicine. 1976, 17 (2): 146-149.
therapy for hyperthyroidism in the first trimester of pregnancy. Journal of Nuclear Medicine. 1976, 17 (2): 146-149.