Abstract
In this study, the induced defects and modifications enhanced by alpha particle on CdO and ZnSe-doped polymethyl methacrylate (PMMA) were investigated. Casting method was used to prepare three sets of thin polymeric sheets doped with variable concentrations of the selected fillers. The prepared CdO and ZnSe particles were in the range 3–21 nm. The samples were irradiated with 4.5 MeV α-particle emitted from 241Am radioactive source at gradually increased fluence. The enhanced change that occurred in the physical properties for both types of samples due to α-particle irradiation was measured using Fourier transform infrared (FTIR), X-ray diffraction (XRD), ultraviolet–visible spectroscopy (UV–Vis), photoluminescence (PL) spectroscopy. All the pristine samples exhibited two separated direct band gaps around 4.0 eV and 4.6 eV, while the irradiated samples showed decreasing for the first band gap up to 3.71 and the second gap remained almost unchanged. The three investigated sets displayed PL emission peaks within the range 270–700 nm. The intensity of the PL peaks was increased by increasing the filler concentration. The net PL was quantified using the area under the emission peaks which showed that α-particle causes interchangeable defects and cross-linking processes. The formation of C–H and O–H function groups due to α-particle interactions was confirmed by FTIR analysis. The results revealed that the filler–polymer interface has a great impact on the formed defects which control the observed characteristics in the polymeric composite medium. The presented data are very helpful for α-particle dosimetric applications using these types of polymeric composites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polymeric materials and their composites found to be sensitive to several types of ionizing radiation such as γ-ray and α-particle. The enhanced effects that occurred due to radiation interaction with polymers have been investigated by many researchers worldwide [1,2,3]. For example, the polymeric nuclear track detectors, such as polyallyl diglycol carbonate (PADC or CR-39), are affected by incident charged particles. They leave permanent trails of damage which could be observed under an ordinary optical microscope after suitable enlargement by etching the medium [4]. The energy released by incident radiation may cause electronic excitation or ionization process depending on the type and energy of radiation and the sensitivity of the target material [5,6,7,8]. Various chemical reactions may lead to breaking bonds, carbon-centered radicals formation, chain scission and cross-linking in polymeric material. This, in turn, tends to modify the structure and optical properties of polymers [9, 10]. The quantity of enhanced changes occurs in the target medium because radiation depends on the energy deposited per unit track length which is known as linear energy transfer (LET) [10]. Through the alpha-particle passage in polymer, a damage track zone is created which is full of free active radical and clusters (number of carbon atoms per cluster). These clusters were supposed to be carriers of electrical conductivity in ion irradiated polymers and responsible for the changes that occur to optical properties [11, 12]. A previous study showed that the cluster size is inversely proportional to the optical energy band gap [13].
Development of several types of polymeric-based nanomaterials for industrial and medical applications is in progress to achieve high-performance and economic fabrication [14, 15]. The recent literature reported novel methods for preparation of polymeric blends containing nano-sized metal oxides or organic molecules with an extensive study on the molecular and morphological structures of the formed phases and their enhanced characteristics [16,17,18]. Most of the reported results focus on interaction of the incorporated nanomaterials with the surrounding polymer chains which modify its physical properties [19]. Metallic nanoparticles showed fluorescence and unconventional optical properties when excited by incident electromagnetic waves. The fluorescence is increased whenever its position becomes close to fluorophores which depends on the morphology and reactivity of such nanoparticles [20, 21].
The metal oxides such as CuO and CdO showed attractive optical and electrical properties in some polymeric-based composites [17, 22]. Similarly, Zn compounds have potential in semiconductor and optical materials [18, 23, 24]. One of the famous compounds is ZnSe, which attracted many researchers to use it in energy conversion devices [25,26,27,28,29]. Both CdO and ZnSe were not tested in heavy ion irradiation when incorporated in PMMA. Due to different color appearance of CdO and ZnSe, it is possible to fabricate wide variety of chromatic composites from each of them as radiation-sensitive materials. Therefore, in this work we pay attention to the effect of α-particle on the surface of two selected polymeric base nanofillers; those are CdO and ZnSe-doped PMMA. With the aid of several analytical techniques such as UV, FTIR and PL spectroscopy it is possible to investigate any changes occur in the samples structure or physical properties due to irradiation. The obtained results may help in using the prepared samples as radiation monitor for α-particle within the studied energy range and fluences.
2 Experimental
PMMA/CdO and PMMA/ZnSe polymeric composite sheets were prepared with different concentrations of 0.3, 1.2 wt% and 1, 3 wt% for CdO and ZnSe, respectively. Nano-CdO, ZnSe were synthesized as given in ref. [22, 30]. The primary materials and solvents were purchased from Sigma-Aldrich, with a purity ≥ 98%. The preparation of polymeric composite thin sheets was done using the solution casting method [22, 31, 32]. The final form of PMMA composites was obtained after drying, and then, they were cut into rectangular thin sheets with an average dimension of 1 × 2 cm and a thickness of 20 µm. The samples were assigned as given in Table 1:
The three investigated sample sets were normally irradiated in air for different irradiation times by α-particles emitted from 9 µCi 241Am source with 4.5 MeV at a distance 1 cm from the source as shown in Fig. 1.
The incident flux (φ) was calculated using the following equation: φ = A/4πr2, where A is the activity of the 241Am source in Bq and r is the distance between the source and the irradiated sample in cm. The total number of emitted α-particles incident on the sample per unit area in a specific irradiation time t, is given by Nα = φ.t [33]. Several irradiation times were taken corresponding to variable fluences of 3 × 108–5 × 109 particle/cm2.
The structure of the incorporated powders, CdO and ZnSe, was characterized by X-ray diffractometer type X-lab Shimadzu X—6000, with Cu-Kα radiation (λ = 0.1546 nm). The particle size was measured using HR-Transmission Electronic Microscope (TEM-JEOL) model (JEM-2100 operating at 200 kV). The chemical structure for the all prepared sheets was investigated with FTIR spectrometer (model Nicolet IS 10, with accuracy 0.4 cm−1) within the range 4000–400 cm−1. The optical properties were studied using double-beam spectrophotometer; model SHIMADZU UV-1061, in the range 190–800 nm (with wavelength resolution ± 0.5 nm). The photoluminescence (PL) characteristics were investigated with a Cary Eclipse Fluorescence Spectrometer. The excitation wavelength was selected after critical scanning within the expected range for each sample, and the PL emission was registered by photomultiplier through a grating monochromator. The excitation and emission slits were adjusted to be 5 and 10, respectively, to avoid detector saturation.
3 Results and discussion
3.1 XRD and HR-TEM analysis
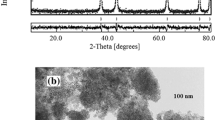
Figure 2 shows the measured X-ray spectra for CdO and ZnSe nanofillers. Both spectra confirmed the formation of pure phases according to the standard cards JCPDS-05-0640 and JCPDS-01-088-2345 [34, 35]. The peak positions were found at 2θ = 27.2°, 45.2°, 53.6°, 65.8° and 72.6°, which were indexed to (111), (220), (311), (400) and (331) planes, respectively. The lattice parameter (a) was calculated for the peak (111) from the relation \({a}_{hkl}={d}_{hkl}\sqrt{{h}^{2}+{k}^{2} +{l}^{2}}\), where (\(h,k,l\)) are miller indices for the diffraction plane, d is the interplanar distance, λ is the X-ray wavelength, and θ is the diffraction angle, and it was found to be (a = b = c = 5.67 Å). The crystal size was determined using the Debye–Scherrer formula D = 0.9 λβ Cos θ, where λ is the wavelength of X-rays (1.5406 Å for Cu kα), θ is the Bragg's angle; β is the full width at half maximum of the (111) peak. The obtained value was 59.32 nm which is close to that reported in Refs. [35, 36].
The particle shape and size of CdO and ZnSe were investigated using their corresponding TEM images. The obtained results for CdO were similar to those published in Ref. [22], where particles showed a hexagonal shape with an average size of 3 nm. Figure 3 shows the image of ZnSe particles under the transmission microscope, which appeared as a semi-rod shape with 20 nm thickness, and was found to be similar to that in ref. [37].
The samples of ZnSe-doped PMMA were visually investigated using an optical scanner, as presented in Fig. 4. The surface of CdO/PMMA samples appeared as that presented in Ref. [22]. The color degradation with concentration for ZnSe was different from that of CdO due to its lower density. The prepared samples allowed different varieties of color appearance, ranging from reddish brown for composites of CdO and light yellow for that of ZnSe. The visual color of each sample was not changed by α-particle irradiation due to its low penetration power compared with γ-ray.
3.2 UV spectroscopic analysis
3.2.1 UV Spectra of pristine samples
The UV spectra for both CdO/PMMA (0.3 and 1.2 wt%) and ZnSe/PMMA (1 and 3 wt%) samples were measured before irradiation and compared with that for pure PMMA sample as shown in Fig. 5a, b. The absorption and transmission spectra showed typical profiles of the polymeric base as reported for PMMA in Refs. [22, 38, 39]. Slight shifts in the position of the absorption band around 270 nm were noticed after the incorporation of CdO and ZnSe in the polymer as well as increasing the absorption intensity. The absorption band appeared at 525 nm for the 1.2 wt% CdO sample which was attributed to CdO effect [22, 40]. In general, the PMMA/CdO samples showed higher absorption intensity than that of PMMA/ZnSe due to the difference in the densities of CdO and ZnSe which affected their transparency inside the polymer. The optical visibility of the investigated samples permits an interactive process with electrons in the filler atoms which are useful for some applications in optoelectronics, data storage and solar cells [41]. The previously reported theoretical modeling of polymeric composite molecules could help in explaining the electronic transitions and optical properties of similar compositions [42, 43].
We calculated Urbach energy (EU) because it is an important parameter governed by the structural disorders, defects, stoichiometric imperfection and surface morphology. Eu is defined as an indicator of the width of localized states in the band gap, which is affected by the material structure. Moreover, it had a direct relation with the optical transition between valence and conduction band and the density inside the states [44, 45]. The formula \(\alpha \mathrm{h}\nu =\beta \mathrm{exp}(\frac{\mathrm{h}\nu }{{\mathrm{E}}_{\mathrm{U}}})\) was used for calculation of Urbach tail, where β is an optical constant, α is the absorption coefficient, and hν is the photon energy. EU was determined by the reciprocal slope of the linear part of ln(α) vs. (hν) graph as shown in Fig. 6a–c for some selected samples as an example. It was found that the calculated Urbach energy (Eua) was ranged from 0.12 eV for the pure PMMA to 0.34 eV for CdO/PMMA and 0.47 eV for ZnSe/PMMA composites. This revealed that the incorporation of polymer with solid nanoparticles increased the width of energy states which reflected the disorder occurred in the band structure of the polymeric matrix and consequentially the increment in amorphous portion [46]. There is good agreement between the determined values of Urbach energy in this work and that reported for other types of polymer including PMMA [47,48,49]. The obtained results are given in Table 5.
3.2.2 UV Spectra of irradiated samples
A primary irradiation test was done to check the sensitivity of the samples to α-particle irradiation. A composite sample of ZnSe/PMMA with 1 wt% was exposed to α-particle for 2 and 18 days as a medium and long irradiation periods, respectively. The UV spectra were measured post-irradiation as shown in Fig. 7. It was found that absorption of the sample was decreased to minimum values after 2 days and the longer irradiation time has no effects. This indicated that the prepared samples were sensitive for the low α-particle fluences within irradiation time of 48 h. Therefore, we designed our experiment to be limited with this time to estimate the gradual enhanced changes in the investigated samples.
Figures 8a–j show the UV spectra for all the investigated samples after irradiation with different α-particle fluences. The absorption bands exhibited slight shift in their positions, and their absorbance decreased with increasing the irradiation fluence. The pure PMMA sheet showed increasing in its absorbance from 38 to 54% with increasing the irradiation dose and slight red shift was observed in its spectrum (Fig. 8a). The same behavior was reported for γ-ray irradiated PMMA and was explained in view of enhanced changes in the localized states [22, 50]. The spectra of Set#2 (PMMA/CdO) and Set#3 (PMMA/ZnSe) samples revealed that they were more sensitive to α-irradiation than Set#1, for high doping concentrations. For example, the composite sample with 1.2 wt% CdO showed clear variation in its absorption bands position and intensities as demonstrated in the incite of Fig. 8c and e. The same behavior was observed for the PMMA/ZnSe sample as presented in Fig. 8g and i, with irregular variation in their absorption intensity below and above that of pristine one. This could be attributed to various defects occurred by α-particle interaction with polymer-nanofiller chains which led to scission and/or cross-linking processes [51, 52]. Conjugated system of bonds [53,54,55] and formation of carbon-enriched cluster or free radicals [56,57,58] were also possible. The variation in the band gap might confirm those induced defects as will be explained below.
The direct band gap Eg of the investigated samples was calculated from the Tauc formula [59, 60] by plotting (αhν)1/2 vs. (hν) as shown in Fig. 9. The results revealed that there were two transition bands, assigned as a and b, for all the investigated samples [22, 32, 61]. Table 2 lists the calculated values of Eg(a) and Eg(b). The major behavior of the band gap Eg(a) was slightly decreased with increasing α-fluencies. This might be attributed to factors affected by both α-interaction with polymer chain and the type of incorporated powders. The more defects occurred in the prepared sheets, the more excitations happened between energy states and formation of more localized states. The decreasing of band gap was attributed to localized states or carbon clusters created by alpha particles upon irradiation [5, 22].
The relation between the band gap values and irradiation time is presented in Fig. 10. One can notice that the sample with 1.2 wt% CdO and 3 wt% ZnSe was highly sensitivity to alpha irradiation in comparison with the other investigated samples. Furthermore, the Set#3 samples showed very low sensitivity, while pure PMMA (Set#1) showed irregular trend of Eg with alpha irradiation. Table 3 presents the linear correlation between changes in the band gap and their corresponding irradiation time. The value of the determined slope was considered as indicator for the sample sensitivity for irradiation.
The number of induced carbon clusters (M) created after alpha irradiation was calculated using the formula (34.3/Eg)2 [62] for all the investigated samples, and the obtained results are listed in Table 4. There was a major increment in the carbon atoms per clusters (M) with increasing α-particle fluence, which might be attributed to the defects and disordered states at or near the conduction band. The values were in a good agreement with that reported in refs. [59, 63, 64].
The Urbach tails for the three sets of the irradiated samples were determined, and the results are presented in Table 5. There was major increment in EU with increasing α-particle fluence, but there was no clear trend or mutual correlation between both parameters. This could be attributed to the defects and disordered states at or near the conduction band [65, 66].
3.3 Photoluminescence analysis
Photoluminescence (PL) spectroscopy is a distinctive technique for studying the distortion present in crystal lattice which forms optical centers. This can be due to the presence of an impurity atom or lattice imperfection. PL spectrum can be used for the prediction of atomic configurations even at concentrations of less than ten in a billion carbon atoms [67, 68]. In the present work, the measured PL spectra of the three investigated sets of samples displayed broad emission peaks in the range 270–700 nm as shown in Fig. 11a and b. The experimental scanning of the excitation wavelengths led to an optimal value at λex = 255 nm. The second-order emission peak found in the range 480–550 nm was discarded. It was noticed that there are three emission peaks assigned as A (275–390 nm), B (412–433 nm) and C (580–680 nm) in Fig. 11a and b. Although the PL spectra are composite dependence, one can find some similarity between emission peaks of the current investigated samples and those for other metallic-doped polymers [69]. The transition energy gap could be calculated using the centroid wavelength of the main emission peak A (275–390 nm) as given in Table 6. The results were consistent with that obtained from UV spectroscopic analysis. This confirmed the high probability of photoelectrons recombination.
It was noticed that for both composites, the peaks intensities were increased with increasing the doping percentage. This reflected the distortion that occurred in the polymeric matrix due to different types of defects and optical centers. As a result, more photoelectrons were excited by the incident light photons which in turn recombined with high emission intensity. It was also noticed that Set#3 emission is higher in their intensity than that of Set#2, which could be due to the excellent luminescence of ZnSe nanofiller in comparison with CdO [70, 71].
The emission spectra from pure CdO and ZnSe powder were also measured at an excitation wavelength of 380 nm, and the results are presented in Fig. 11C. The spectra exhibited three emission peaks at 425, 488, 518 nm for CdO and 469, 483, 526 nm for ZnSe. Those peaks were attributed to near-band edge (NBE) and deep level (DL) emissions [72,73,74,75,76]. The former is responsible for emission in the form of UV up to blue green regions, while the latter causes emission in the green region starting from 510 nm. Both emission bands were affected by preparation conditions, impurity levels and structural defects of the prepared powder [77, 78]. The same peaks could be characterized in the composites spectra shown in Fig. 11a and b, but with very low intensity due to low filler concentrations. Furthermore, the major polymeric PL profile changed with increasing the doping concentration with slight blue shifts in the peak positions. The results were in agreement with the fact that CdO/PMMA composite had heterogeneous structure as previously reported in ref. [22]. The same behavior of ZnSe/PMMA was due to the same reason. The physical bilateral junction between the nanopowder and the polymeric matrix could be explained using suggested mechanisms of particle distribution as found in refs [22, 60, 79,80,81]. A previous study on luminescence behavior showed that the intermolecular conjugation might lead to decreasing the excitation probability of the delocalized π-electrons in the composite [82]. The aggregation, in turn, results in a shorter conjugated molecular system which appeared as blue shift in the emission spectra.
The area under the characteristic emission peak and its height were plotted vs. doping concentration as shown in Fig. 12. It is obvious that the emission peaks area and the height increased gradually as the powder concentration increased.
According to the calculated range of 4.5 MeV α-particle in PMMA, its interaction effect was limited to 40–60 µm from the exposed surface, while the other side of the sample was unaffected. We checked this point by measuring both irradiated and unirradiated surfaces for the same sample as shown in Fig. 13. The results confirmed the radiation effect of α-particle on the sample surface.
The PL of all irradiated samples was measured, and the obtained spectra were compared with that of unirradiated ones as shown in Fig. 14a–c. To detect significant variation in the PL spectra of the investigated samples, the highest concentration samples were considered in addition to that of pure PMMA for comparison. The emission peaks of pure PMMA (Fig. 14a) showed gradual increases in their intensities with increasing the irradiation fluence up to 1.15 × 109 cm−2 and, thereafter, recognizable decrease in the peak intensity as well as distortion in the spectrum profile at maximum fluence (4.58 × 109). This enhanced change might be due to the ionization effects of the incident α-particles, which may charge density and radiative energy states in the band gap region [83]. The variable response of the samples to radiation may reflect the various types of defects or generation of localized chemical species on the sample surface.
To quantify the enhanced changes in the PL emission, we determined the area under the main PL peak and its height for all the investigated samples with increasing the α-particle fluence as represented in Fig. 15a–d. The results for pure PMMA were presented for comparison as dark square points in all the figures. It was noticed that the integrated PL intensity for pure PMMA had maximum value around the fluence of 1 × 109/cm2. On the other hand, the 1.2 wt% CdO sample had a linear behavior with increasing α-particle fluence. The obtained results for PMMA/ZnSe samples should wide hump profile around the fluence of 1.5 × 109/cm2 in constant with PMMA behavior. The explanation of those different varieties in changes of PL could be due to the filler type and its distribution inside the polymeric matrix. However, as discussed above in the UV analysis the interaction of α-particle with the composite as a whole could lead to defects or cross-linking, which affect its inherent PL characteristics.
3.4 IR spectroscopic analysis
Figure 16 shows the IR spectra for the investigated pure PMMA and both types of its composites with maximum doping concentration (1.2 wt% for CdO) and (3 wt% for ZnSe) for comparison. The characteristic peaks of the functional groups were assigned as given in Table 7, which is in agreement with that found in Refs. [22, 70, 84,85,86,87,88]. The main characteristic peaks PMMA were at positions 1726 and 1140 cm−1, and they were presented in all composites as a basic profile for the polymeric sheets. There were two opposite effects on the intensities of those two main bands as indicated on the spectrum. CdO-doped PMMA had a lower intensity than that for pure polymer, while PMMA/ZnSe has a higher intensity. This might be due to interactions between the filler nanoparticles with the polymer bonds as previously reported in other similar study on InO/PMMA composite [89]. Furthermore, one can notice the significant changes in the characteristic band intensity for Cd–O in the range 400–1200 cm−1 due to CdO.
The FTIR spectra of the α-particle irradiated samples are shown in Fig. 17 for pure PMMA, PMMA/CdO (1.2 wt%) and PMMA/ZnSe (3 wt%). The obtained spectra were compared with that for similar unirradiated samples. It was noticed that the spectral lines were at the same position and no shifts occurred due to irradiation.
There were noticeable variations in the intensities of the characteristic bands, which was more significant in the high doses of irradiation (2.3–4.6 × 109/cm2). The influence of the polymeric bonds seemed to be different at low irradiation doses (3 × 108–1 × 109) than at higher ones (> 1 × 109). Furthermore, the spectrum of PMMA/CdO (1.2 wt%) showed more sensitivity to radiation in the range 480–850 cm−1. According to previous study on He+ ions, irradiation of polymeric composite showed that peak heights decreased upon irradiation due to chain scission. Other evidence such as peak shifts in the methyl groups (2940, 2910 cm−1) to methylene groups (2927, 2867 cm−1) was due to cross-linking behavior. To follow up the irradiation effects on the C=O and C–O–C bands at 1726 and 1140 cm−1, respectively, a representation between the peak height vs. the fluence was done as shown in Fig. 18. The behavior of irradiated samples was in agreement with that obtained by PL study (see Fig. 15). It should be noted that the alpha particle had surface effect due to its low energy and this confirmed that the bulk of the sample was very stable. This might also explain the competition between the scission and cross-linking processes by different types of processes, such as group degradations, adsorption of oxygen in the air, active sites and free radicals during irradiation. The recovery of broken bonds was also possible which allowed reverse sensitivity to radiation. Moreover, it was known that metal oxides had higher surface energy (strong molecular attraction) and polarity than polymeric medium, which explained the majority of polymeric characteristics without formation of a new composite phase [22].
4 Conclusion
Two types of PMMA composites incorporated with CdO and ZnSe nanofillers were synthesized. The samples were homogeneously fed by both CdO and ZnSe with controlling their feeding concentration. The observed evidences on the physical characteristics for PMMA/CdO and PMMA/ZnSe samples revealed that α-particle caused effective degradation in a thin layer of the surface (40–60 µm) due to its low penetration power. The major trend of the band gap values confirms the formation of carbon clusters and localized states due to α-interaction which caused major decrease in the band gap. This effect is enhanced by increasing the incorporation density of the nanofillers, specially for CdO. Increasing of the PL intensity with increased alpha-particle fluences confirmed the formation of new radiative recombination levels in the forbidden energy gap. The luminescence behavior could be explained in view of changing the electronic structure and molecular binding defects which appeared as blue shift in the emission spectrum of some of the irradiated samples. The observed sensitivity of the prepared samples for α-particles found to be dependent on the filler concentration. With choice of optimum filler concentration, the samples could be act as good candidates for α-particle detector by changing their optical characteristics.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
A. Ashfaq, M. Clochard, X. Coqueret, C. Dispenza, M.S. Driscoll, P. Ulanski, M. Al-Sheikhly, Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 12, 2877 (2020)
M. Rahaman, G. Periyasami, A. Aldalbahi, Effect of different gamma dose and chemical etching on pre-and post-alpha-irradiated PM-355 polymer. Int. J. Polym. Sci. 8825079, 1–9 (2021)
M. El Ghazaly, M.H. Nabil, Characterization of saturation of CR-39 detector at high alpha-particle fluence. Nuclear Eng. Tech. 50, 432–438 (2018)
R. Ilić, S.A. Durrani, Solid state nuclear track detectors, in Handbook of radioactivity analysis by M. L'Annunziata 2nd edition, Netherlands: Elsevier Science Publishers (2003).
T. Al-Naggar, B. El-Badry, N. Abdel All, Study the modifications induced by alpha particles in cellulose nitrate NTD. Vacuum 160, 31–36 (2019)
Q. Chen, T. Hajagos, Q. Pei, Conjugated polymers for radiation detection. Annu. Rep. Prog. Chem Sect. C 107, 298–318 (2011)
J. Ball, D. Adrian, Moore, S. Turner, Essential physics for radiographer, 4th edn. (Wiley-Blackwell, Hoboken, 2012)
M. Eissa, Effect of transferred electronic energy density on optical, electrical and structural properties of Polyallyl-Diglycol Carbonate (CR-39) Polymer. J. Macromol. Sci. Part B Phys. 53, 529–540 (2014)
IAEA-TECDOC-1617, Controlling of degradation effects in radiation processing of polymers, 2009, Vienna, Austria.
M. Eissa, M. Kaid, N. Kamel, Study of the effects of low and high linear energy transfers on Poly(methyl-methacrylate) samples. J. Appl. Polym. Sci. 125, 3682–3687 (2012)
A. Negi, A. Semwal, S. Chandra, R. Hariwal, R. Sonkawade, D. Kanjilal, J. Rana, R. Ramola, Modifications induced by Li+3, Ni+9 and Au+9 ion beams to CR-39 polymer track detector. Radiat. Meas. 46, 127–132 (2011)
D. Fink, M. Miller, L. Chadderton, P. Cannington, R. Elliman, D. Mc Donald, Optically absorbing layers on ion beam modified polymers: a study of their evolution and properties. Nuclear Instrum. Methods B 32, 125–130 (1988)
D. Fink, W. Chung, R. Klett, A. Schmoidt, J. Cardosa, R. Montiel, M. Vazqez, L. Wang, F. Hosoi, H. Omichi, P. Goppelt-Langer, Carbonaceous clusters in irradiated polymers as revealed by UV-visible spectrometry. Radiat. Eff. Defects Solids 133, 193–208 (1995)
B. Shekar, S. Sathish, B.T. Bhavyasree, B. Ranjith Kumar, Characterization of nano scale PMMA films prepared by FDC technique for FET applications. Adv. Mater. Res. 678, 309–315 (2013)
A.A.I. Abd-Elmageed, S.M. Ibrahim, A. Bourezgui, A.F. Al-Hossainy, Synthesis, DFT studies, fabrication, and optical characterization of the [ZnCMC]TF polymer (organic/inorganic) as an optoelectronic device. New J. Chem. 44, 8621–8637 (2020)
S.M. Ibrahim, A.F. Al-Hossainy, Synthesis, structural characterization, DFT, kinetics and mechanism of oxidation of bromothymol blue: application to textile industrial wastewater treatment. Chem. Pap. 75, 297–309 (2021)
S.M. Ibrahim, A. Bourezgui, A.F. Al-Hossainy, Novel synthesis, DFT and investigation of the optical and electrical properties of carboxymethyl cellulose/thiobarbituric acid/copper oxide [CMC + TBA/CuO]C nanocomposite film. J. Polym. Res. 27, 264 (2020)
S.M. Ibrahim, A. Bourezgui, A.A.I. Abd-Elmageed, I. Kacem, A.F. Al-Hossainy, Structural and optical characterization of novel [ZnKCMC]TF for optoelectronic device applications. J. Mater. Sci.: Mater. Electron. 31, 8690–8704 (2020)
S.K. Sharma, J. Prakash, K. Sudarshan, D. Sen, S. Mazumder, P.K. Pujari, Structure at interphase of Poly (vinyl alcohol)–SiCNanofiber Composite and its impact on mechanical properties: positron Annihilation and Small-Angle X-ray Scattering Studies. Macromolecules 48(16), 5706–5713 (2015)
I.H. El Azab, HKh. Thabet, Sh.A. Almotairi, M.G.A. Saleh, R.T. Mogharbel, S.A. Mahmoud, A.A. El-Rayyes, A. Ibrahim, MSh. Zoromba, M.H. Abdel-Aziz, S.M. Ibrahim, A.F. Al-Hossainy, Synthesis of a novel coumarin heterocyclic derivative and fabrication of hybrid nanocomposite thin film with CoOFe 2 O 4 for optoelectronic applications. J. Mol. Struct. 1241, 130640 (2021)
O. Mirzov, I.G. Scheblykin, Photoluminescence spectra of a conjugated polymer: from films and solutions to single molecules. Phys. Chem. Chem. Phys. 8, 5569–5576 (2006)
D. El-Malawy, M. Al-Abyad, M. El Ghazaly, S. Abdel Samad, H. Hassan, γ-ray effects on PMMA polymeric sheets doped with CdO nano particles. Radiat. Phys. Chem. 184, 109463 (2021)
H. Zhang, M. Chen, Two-dimensional β-phase group-VA binary compounds for versatile electronic and optical properties. J. Mater. Chem. C 6, 11694–11700 (2018)
M. Pumera, Z. Sofer, 2D monoelemental arsenene, antimonene, and Bismuthene: beyond black phosphorus. Adv. Mater. 29, 1605299 (2017)
K. Ou, S. Wang, M. Huang, Y. Zhang, Y. Wang, X. Duan, L. Yi, Influence of thickness and annealing on photoluminescence of nanostructured ZnSe/ZnS multilayer thin films prepared by electron beam evaporation. J. Lumin. 199, 34–38 (2018)
D. Hile, H. Swart, S. Motloung, L. Koao, Zinc selenide semiconductor: synthesis, properties and applications, Nanoscale Compound Semiconductors and their Optoelectronics Applications, Wood head Publishing Series in Electronic and Optical Materials (2022)67-84
S. Chengcheng, G. Yarong, W. Weijia, Z. Lijuan, ZnSe based semiconductor core-shell structures: from preparation to application, NASA Astrophysics Data System (ADS). Opt. Mater. 81, 12–22 (2018)
S.K. Hong, E. Kurts, J.H. Chang, T. Hanada, M. Oku, T. Yao, Effect of Zn precursor on structural, morphological and optical properties of ZnSe nanoparticles. Appl. Phys. Lett. 78, 165 (2001)
H. Haiyan, Y. Xi, W. Minqiang, Preparation and optical characteristics of ZnSe nanocrystals doped glass by sol gel in situ crystallization method, NASA astrophysics data system (ADS). Opt. Mater. 29, 573–577 (2007)
H. Hassan, K. El-Azony, A. Azza, S. Qaim, Investigation of selenium compounds as targets for 76,77Br production using protons of energies up to 34 MeV. Radiochim. Acta 105, 841–850 (2017)
M. El Ghazaly, Comparison between different models for alpha-particle range determination and a new approach to Cr-39 detector. J. Korean Phys. Soc. 61, 336 (2012)
M.S. Refat, M. Eltabey, I. Ali, H. Hassan, Optical and electrical characteristics of thin PMMA sheets doped with Cu-Zn ferrite nanoparticles. Appl. Phys. A 229, 126 (2020)
A. Ridha, N. Kadhim, N. Mohammed, Correlation between the track density and absorbance of alpha particles using CR-39 detectors from UV–visible spectrum. J. Phys. Sci. 30, 37–49 (2019)
M. Abbas, W. Tawfik, J. Chen, CdO nanorods and Cd(OH)2/Ag core/ satellite nanorods: rapid and efficient sonochemical synthesis, characterization and their magnetic properties. Ultrason. Sonochem. 40, 577–582 (2018)
A. Ahamed, K. Ramar, P. Kumar, Synthesis and characterization of ZnSe nanoparticles by co-precipitation method. J. Nanosci. Technol. 2, 148 (2016)
K. Senthilkumar, T. Kalaivani, S. Kanagesan, V. Balasubramanian, Synthesis and characterization studies of ZnSe quantum dots. J. Mater. Sci. Mater Electron 23, 2048–2052 (2012)
D. Yuvaraj, R. Kumar, V. Selvan, M. Sathyanarayanan, K. Rao, Growth of ZnSe nano and microstructures at high vacuum by thermal evaporation. Appl. Nano Sci. 4, 469 (2014)
A. Haider, Z. Al-Shibaany, R. Hawy, N. Hamed, Impact of PS/PMMA polymer ratios with nanocomposite material on optical and morphological properties. Ziggurat J. Mater. Technol. 1, 1–10 (2020)
Y. Lin, J. Zhang, E. Kumacheva, Nonlinear optical figures of merit of processible composite of poly(2-methoxy,5-(2’-(ethyl)hexyloxy)-p-phenylenevinylene) and poly(methyl methacrylate). J. Appl. Phys. 91, 522 (2002)
T. Xaba, M.J. Moloto, N. Moloto, Bis(2-hydroxy-1-naphthalenehydrato) metal complexes as source of face-centered-cubic trioctylphosphine oxide-capped ZnO and CdO nanoparticles using oleylamine as dispersion medium. Asian J. Chem. 28, 1015 (2016)
A.F. Al-Hossainy, MSh. Zoromba, Doped-poly (para-nitroaniline- co-aniline): Synthesis, semiconductor characteristics, density, functional theory and photoelectric properties. J. AlloyS Compd. 789, 670–683 (2019)
A.F. Al-Hossainy, A. Ibrahim, The effects of annealing temperature on the structural properties and optical constants of a novel DPEA-MR-Zn organic crystalline semiconductor nanostructure thin films. Optic. Mater. 73, 138e153 (2017)
A.F. Al-Hossainy, M.R. Eid, MSh. Zoromba, Structural, DFT, optical dispersion characteristics of novel [DPPA-Zn-MR(Cl)(H2O)] nanostructured thin films]. Mater. Chem. Phys. 232, 180–192 (2019)
N. Galkin, Syntheses and structural control of silicide, oxide and metallic nano-structured materials. Solid State Phenom. 213, 35–41 (2014)
A. Dhanaraj, K. Das, J. Keller, A study of the optical band gap energy and urbach energy of fullerene (c60) doped PMMA nanocomposites. AIP Conf. Proc. 2270, 110040 (2020)
S. Chiad, N. Habubi, M. Abdul-Allah, Determination the dispersion parameters and urbach tail of iron chromate doped PMMA films, Iraqi. J. Phys. 10, 12–17 (2012)
S. Aziz, O. Abdullah, A. Hussein, H. Ahmed, From insulating PMMA polymer to conjugated double bond behavior: green chemistry as a novel approach to fabricate small band gap polymers. Polymers 9, 626 (2017)
S. Aziz, H. Ahmed, A. Hussein, A. Fathulla, R. Wsw, R. Hussein, Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci.: Mater. Electron. 26, 8022 (2015)
S. Prasher, M. Kumar, S. Singh, Electrical and optical properties of O6+ ion beam–irradiated polymers. Int. J. Polym. Anal. Charact. 19, 204 (2014)
M.F. Al-Kadhemy, Z.S. Rasheed, S.R. Salim, Alpha -ray irradiation effect on optical properties for coumarin doped polystyrene polymer films. Int. J. Chem Tech Res. 11, 277 (2018)
R. Kumar, P. Singh, UV–visible and infrared spectroscopic studies of Li3+ and C5+ irradiated PADC polymer. Results Phys. 3, 122 (2013)
P. Singh, R. Kumar, Study of structural and free volume properties of swift heavy ion irradiated Polyallyldiglycol carbonate polymer films. Vacuum 96, 46 (2013)
T. Sharma, S. Aggarwal, A. Sharma, S. Kumar, Effect of nitrogen ion implantation on the optical and structural characteristics of CR-39 polymer. Appl. Phys. 102, 063527 (2007)
V. Kumar, R.G. Sonkawade, S.K. Chakarvarti, P. Singh, A.S. Dhaliwal, Carbon ion beam induced modifications of optical, structural and chemical properties in PADC and PET polymers. Radiat. Phys. Chem. 81, 652 (2012)
P. Singh, R. Kumar, R. Prasad, Free volume evolution in 50 MeV Li3+ ion-irradiated polymers studied by positron annihilation lifetime spectroscopy. Radiat. Eff. Defects Solids 168, 97 (2013)
S. Gupta, D. Choudhary, A. Sarma, Study of carbonaceous clusters in irradiated polycarbonate with UV–Vis spectroscopy. J. Polym. Sci. B38, 1589 (2000)
D. Sinha, T. Phukan, S.P. Tripathy, V. Mishra, K.K. Dwivedi, Optical and electrical properties of gamma irradiated PADC detector. Radiat. Measurement 34, 109 (2001)
R.K. Dhillon, P. Singh, S.K. Gupta, S. Singh, R. Kumar, Study of high energy (MeV) N6+ ion and gamma radiation induced modifications in low density polyethylene (LDPE) polymer. Nucl. Instr. Meth. B301, 12 (2013)
Z.K. Heiba, M.B. Mohamed, N.Y. Mostafa, A.M. El-Naggar, Structural and optical properties of Cd1−xMnxFe2O4/PMMA nanocomposites. J. Inorg. Organomet. Polym Mater. 30, 1898 (2020)
S.M. Al-Sofiany, H.E. Hassan, Radiation treatment of Cd2SnO4 thin films prepared by RF sputtering. J. Alloys Compd. 651, 149 (2015)
H.E. Hassan, M.S. Refat, T. Sharshar, Optical and positron annihilation spectroscopic studies on PMMA polymer doped by rhodamine B/chloranilic acid charge transfer complex: special relevance to the effect of γ-ray irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 159, 238–248 (2016)
Y.S. Rammah, E.M. Awad, Modifications of the optical properties for DAM-ADC nuclear track detector exposed to alpha particles. Radiat. Phys. Chem. 146, 42–46 (2018). https://doi.org/10.1016/j.radphyschem.2018.01.004
D.E. Abulyazied, H.M. Abomostafa, G.M. El Komy, Magnetic structured nickel core-shell @silica/PMMA nanocomposites from synthesis to applications. J. Inorg. Organometall. Polym. Mater. 30, 2335 (2020)
Y. Li, P. Miao, W. Zhou, X. Gong, X. Zhao, N-doped carbon-dots for luminescent solar concentrators. J. Mater. Chem. 5, 21452 (2017)
N.F. Mott, E.A. Davis, Electronic processes in non-crystalline materials (Clarendon Press, Oxford, 1979)
A.Y. Abdel-latif, H.M. Kotb, M.M. Hafiz, M.A. Dabban, Influence of heat treatment on the structural, optical and electrical properties of Cd2OSn10Se70 thin films. Mater. Sci. Semicond. Process. 30, 502 (2015)
S. Eaton-Magaña, C.M. Breeding, An introduction to photoluminescence spectroscopy for diamond and its applications in gemology, introduction to photoluminescence spectroscopy. Gems Gemol. 52, 2 (2016)
A. Wotherspoon, J.W. Steeds, B. Catmull, J. Butler, Photoluminescence and positron annihilation measurements of nitrogen doped CVD diamond. Diam. Relat. Mater. 12, 652 (2003)
A.R. Ghazy, M.G. Shalaby, A. Ibrahim, A. ElShaer, Y.A.G. Mahmoud, A.F. Al-Hossainy, Synthesis, structural and optical properties of Fungal biosynthesized Cu2O nanoparticles doped Poly methyl methacrylate -co- Acrylonitrile copolymer nanocomposite films using experimental data and TD-DFT/DMOl 3 computations. J. Mol. Struct. 1269, 133776 (2022)
K. Saikia, P. Deb, E. Kalita, Sensitive fluorescence response of ZnSe(S) quantum dots: an efficient fluorescence probe. Physica Script 87, 065802 (2013)
L. Ding, Z. Peng, P. Zhou, G.J. Cheng, Q. Nian, D. Lin, J. Zhou, Y. Liang, Preparation and effect of lighting on structures and properties of GSH capped ZnSe QDs. J. Fluoresc. 25, 1663 (2015)
S. Balamurugana, A.R. Balua, K. Usharania, M. Suganyaa, S. Anithaa, D. Prabhab, S. Ilangovan, Synthesis of CdO nanopowders by a simple soft chemical method and evaluation of their antimicrobial activities. Pac. Sci. Rev. A 18, 228 (2016)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, Multifunctional properties of CdO nanostructures synthesized through microwave assisted hydrothermal method. Mater. Res. Innov. 23, 310 (2019)
S. Park, S. Kim, H. Ko, C. Lee, Surface plasmon resonance-enhanced luminescence in Pd-functionalized ZnO nanowires. J. Nanosci. Nanotechnol. 15, 5301 (2015)
K. Senthilkumar, T. Kalaivani, S. Kanagesan, V. Balasubramanian, Synthesis and characterization studies of ZnSe quantum dots. J. Mater. Sci.: Mater. Electron. 23, 2048 (2012)
D. Yuvaraj, R. Kumar, V. Selvan, M. Sathyanarayanan, K. Rao, Growth of ZnSe nano and microstructures at high vacuum by thermal evaporation. Appl. Nanosci. 4, 469 (2014)
J. Basu, R. Divakar, J. Nowak, S. Hofmann, A. Colli, A. Franciosi, C.B. Carte, Structure and growth mechanism of ZnSe nanowires. Appl. Phys. 104, 064302 (2008)
U. Philipose, A. Saxena, H.E. Ruda, P.J. Simpson, Y.Q. Wang, K.L. Kavanagh, Defect studies of ZnSe nanowires. Nanotechnology 19, 215715 (2008)
P.C. Papaphilippou, A. Pourgouris, O. Marinica, A. Taculescu, G.I. Athanasopoulos, L. Vekas, T. Krasia-Christoforou, Fabrication and characterization of superparamagnetic and thermoresponsive hydrogels based on oleic-acid-coated Fe3O4 nanoparticles, hexa(ethylene glycol) methyl ether methacrylate and 2-(acetoacetoxy)ethyl methacrylate. J. Magn. Magn. Mater. 323, 557 (2011)
Z. Li, J. Shen, H. Ma, X. Lu, M. Shi, N. Li, M. Ye, Preparation and characterization of sodium alginate/poly(N-isopropylacrylamide)/clay semi-IPN magnetic hydrogels. Polym. Bull. 68, 153 (2012)
N.S. Satarkar, J.Z. Hilt, Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 4, 11 (2008)
M.S. Tolba, A.F. Al-Hossainy, A.M. Kamal Eldean, O. Younis, From blue to green photoluminescence: design, synthesis, and dft calculations of heterocyclic compounds containing chromenothienopyrimidine moiety Asian. J. Org. Chem. 11, e202100801 (2022)
R.A. Rizk, A.M. Abdul-Kader, Z.I. Ali, M. Ali, Effect of ion bombardment on the optical properties of LDPE/EPDM polymer blends. Vacuum 83, 805 (2008)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S.S. Krishnan, Ultrasonic assisted CdO–MgO nanocomposite for multifunctional applications. Mater. Technol. Adv. Perform. Mater. 34, 403 (2019)
S. Kumar, B. Ahmed, A.K. Ojha, J. Das, A. Kumar, Facile synthesis of CdO nanorods and exploiting its properties towards super capacitor electrode materials and low power UV irradiation driven photocatalysis against methylene blue dye. Mater. Res. Bull. 90, 224 (2017)
A.K. Kushwaha, N. Gupta, M.C. Chattopadhyaya, Dynamics of adsorption of Ni(II), Co(II) and Cu(II) from aqueous solution onto newly synthesized poly[N-(4-[4-(aminophenyl) methyl phenyl methacrylamide]). Arab. J. Chem. 10, 1645 (2017)
R.M. Ahmed, Optical study on poly (methylmethacrylate)/poly(vinyl acetate) blends. Int. J. Photoenergy 2009, 7 (2009)
R. Huszank, E. Szilágyi, Z. Szoboszlai, Z. Szikszai, Investigation of chemical changes in PMMA induced by 1.6 MeV He+ irradiation by ion beam analytical methods (RBS-ERDA) and infrared spectroscopy (ATR-FTIR). Nucl. Instrum. Meth. B 450, 364 (2019)
A. Singhal, K.A. Dubey, Y.K. Bhardwaj, D. Jain, S. Choudhurya, A.K. Tyagia, UV-shielding transparent PMMA/In2O3 nanocomposite films based on In2O3 nanoparticles. RSC Adv. 3, 20913 (2013)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Malawy, D., Hassan, H.E., El Ghazaly, M. et al. Low-energy α-particle irradiation of polymeric-based nanofiller. Eur. Phys. J. Plus 138, 759 (2023). https://doi.org/10.1140/epjp/s13360-023-04300-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-04300-x