Abstract
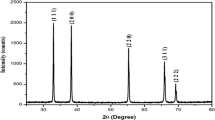
The present work is a systematic study on Cd(OH)2 and CdO nanoparticle synthesized by hydrothermal route. The structural properties are confirmed with standard XRD results of Cd(OH)2 and CdO. Phase transformation: Cd(OH)2 (hexagonal) to CdO (face-centred) is a notable feature observed from the structural parameters. Further, Fourier transform infrared (FTIR), scanning electron microscope (SEM), UV–visible absorption and antibacterial studies were taken for Cd(OH)2 and CdO. The FTIR spectra of Cd(OH)2 show bands of 1404, 850 cm−1 which can be ascribed to the confirmation of Cd–O and 524 cm−1 assigned to Cd–O stretching in the spectra of CdO which correlates with the literature. The morphology of the both the samples shows the shape of nanocubes. Calcination of 400 °C is reflected in the reduced size of the particle in SEM. The optical bandgap of CdO (2.14 eV) is lower than Cd(OH)2 (3.14 eV). The antibacterial evaluation shows that the effect observed was more in CdO than in Cd(OH)2. It was evident that the preparation time of CdONps was economical owing to its simplicity.
Graphic abstract












Similar content being viewed by others
Data Availability Statement
This manuscript has associated data in a data repository. [Authors’ comment: All data included in this manuscript are available upon request by contacting with the corresponding author.]
References
Z. Yang, W. Zhong, Y. Yin et al., Controllable synthesis of single-crystalline CdO and Cd(OH)2 nanowires by a simple hydrothermal approach. Nanoscale Res. Lett. 5, 961 (2010). https://doi.org/10.1007/s11671-010-9589-y
P. Qu, S. Yan, H. Meng, Controllabe growth of cadmium hydroxide nanostructures by hydrothermal method. Solid State Sci. 12(1), 83–87 (2010). https://doi.org/10.1016/j.solidstatesciences.2009.10.008
A. Taufik, H. Tju, S.P. Prakoso, R. Saleh, Different routes of synthesized CdO nanoparticles through microwave-assistedmethods and photocatalytic stud. AIP Conf. Proc. (2018). https://doi.org/10.1063/1.5064032
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, Multifunctional properties of CdO nanostructures synthesised through microwave assisted hydrothermal method. Mater. Res. Innov. (2018). https://doi.org/10.1080/14328917.2018.1475443
N. Shanmugam, B. Saravanan, R. Reagan, N. Kannadasan, K. Sathishkumar et al., Effect of thermal annealing on the Cd(OH)2 and preparation of Cdo nanocrystals. Mod. Chem. Appl. 2, 124 (2014). https://doi.org/10.4172/2329-6798.1000124
A. Tadjarodi, M. Imani, H. Kerdari, K. Bijanzad, D. Khaledi, M. Rad, Preparation of CdO rhombus-like nanostructure and its photocatalytic degradation of azo dyes from aqueous solution. Nanomater. Nanotechnol. 4, 16 (2014)
Z. Guo, M. Li, J. Liu, Highly porous CdO nanowires: preparation based on hydroxy- and carbonate-containing cadmium compound precursor nanowires, gas sensing and optical properties. Nanotechnology. 19, 245611 (2008)
M. Ye, W. Zheng et al., Ultralong cadmium hydroxide nanowires: synthesis characterization transformation into CdO nanostrands. Langmuir 23, 9064–9068 (2007)
W. Xia, Y. Liu, J. Li, C. Chen, Investigation of CdO hexagonal nanoflakes synthesized by a hydrothermal method for liquefied petroleum gas detection. Anal. Methods 8(33), 6265–6269 (2016). https://doi.org/10.1039/c6ay01914e
L.A. Saghatforoush, S. Sanati, R. Mehdizadeh, Hasanzadeh, Solvothermal synthesis of Cd(OH)2 and CdO nanocrystals and application as a new electrochemical sensor for simultaneous determination of norfloxacin and lomefloxacin. Superlattices Microstruct. 52, 885–893 (2012)
S. Balamurugan, A.R. Balu, K. Usharani, M. Suganya, S. Anitha, D. Prabha, S. Ilangovan, Synthesis of CdO nanopowders by a simple soft chemical method and evaluation of their antimicrobial activities. Pac. Sci. Rev. A Nat. Sci. Eng. 18(3), 228–232 (2016). https://doi.org/10.1016/j.psra.2016.10.003
H.M. Rietveld, The Rietveld method. Phys. Scr. 89, 098002–098008 (2014). https://doi.org/10.1002/jps.23909
H.M. Rietveld, A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969). https://doi.org/10.1107/S0021889869006558
D. Sivaganesh, S. Saravanakumar, V. Sivakumar, M. Rajajeyagathan Ramanathan, J. Arunpadian, T.K. NandhaGopal, Thirumalaisamy Surfactant-assisted synthesis of ZnWO4 nanostructures: a view on photocatalystis, photoluminescence and electron density distribution analysis. Mater. Character. 159, 110035–110050 (2020)
V. Petrícek, M. Dušek, L. Palatinus, Crystallographic computing system JANA2006: general features. Zeitschr. Krist. 229, 345–52 (2014). https://doi.org/10.1515/zkri-2014-1737
D. Sivaganesh, S. Saravanakumar, V. Sivakumar, S. Sasikumar, J. Nandha Gopal, R. Ramanathan, ZnWO4:Eu3+ phosphor with intense blue LED excitation: photoluminescence and electron density distribution analysis. Luminescence 36(1), 99–109 (2020). https://doi.org/10.1002/bio.3920
S. Israel, R. Saravanan, N. Srinivasan, S.K. Mohanlal, An investigation on the bonding in MgO, CaO, SrO and BaO from the MEM electron density distributions. J. Phys. Chem. Solids 64, 879–886 (2003). http://ir.mkuniversity.ac.in/id/eprint/2335
D. Sivaganesh, S. Saravanakumar, V. Sivakumar, S. Sasikumar, J. Nandha Gopal, S. Kalpana, R. Ramanathan, Sm3+ induced SrWO4 phosphor: analysis of photoluminescence and photocatalytic properties with electron density distribution studies. J. Mater. Sci. Mater. Electron. 31(11), 8865–8883 (2020). https://doi.org/10.1007/s10854-020-03421-8
K. Momma, T. Ikeda, A.A. Belik, F. Izumi, Dysnomia, a computer program for maximum-entropy method (MEM) analysis and its performance in the MEM-based pattern fitting. Powder Diffract. 28(3), 184–193 (2013)
D. Sivaganesh, S. Saravanakumar, V. Sivakumar, R. Sangeetha, L. John Berchmans, K.S. Syed Ali, A.M. Alshehri, Effect of preparation techniques on BaWO4: Structural, morphological, optical and electron density distribution analysis. J. Mater. Sci. Mater. Electron. 32(2), 1466–1475 (2021). https://doi.org/10.1007/s10854-020-04917-z
K. Momma, F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44(6), 1272–1276 (2011). https://doi.org/10.1107/S0021889811038970
D. Sivaganesh, S. Saravanakumar, V. Sivakumar, K.S. Syed Ali, E. Akapo, Ezra Alemayehu, R. Ramanathan, R. Saravanan, Structural, optical and charge density analysis of Al doped ZnO Materials. J. Mater. Sci. Mater. Electron. 30, 2966–2974 (2019). https://doi.org/10.1007/s10854-018-00574-5
M. Ristic, S. Popovic, S. Music, Formation and properties of Cd(OH)2 and CdO particles. Mater. Lett. 58, 2494–2499 (2004)
B. Weckler, H.D. Lutz, Near-infrared spectra of M(OH)Cl (M = Ca, Cd, Sr), Zn(OH)F, g-Cd(OH)2, Sr(OH)2, and brucite-type hydroxides M(OH)2 (M = Mg, Ca, Mn, Fe Co, Ni, Cd). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 52, 1507–1513 (1996)
M. Schmidt, H.D. Lutz, g-Cd(OH)2, A common hydroxide or an aquoxy-hydroxide. Mater. Res. Bull. 26, 605–612 (1991). https://doi.org/10.1016/0025-5408(91)90103-S
D.J. Jeejamol, CdO nanoparticles: facile synthesis and influence of particle size on photocatalytic degradation of methylene blue. IOSR J. Appl. Phys. 9(5), 66–73 (2017)
Karthik, K., Dhanuskodi, S., Gopinath, C., Sivaramakrishnan, S. Antibacterial activities of CdO microplates synthesized by hydrothermal method. Int. J. Innov. Res. Sci. Eng. 558–561 (2014)
K. Karthik, S. Dhanuskodi, S. Prabukumar et al., Dielectric and antibacterial studies of microwave assisted calcium hydroxide nanoparticles. J. Mater. Sci. Mater. Electron. 28, 16509–16518 (2017). https://doi.org/10.1007/s10854-017-7563-5
K. Karthik, S. Dhanuskodi, C.S. Prabukumar et al., Nanostructured CdO–NiO composite for multifunctional applications. J. Phys. Chem. Solids 112, 106–118 (2018). https://doi.org/10.1016/j.jpcs.2017.09.016
K. Karthik, S. Dhanuskodi, S. Prabukumar et al., Multifunctional properties of microwave assisted CdO–NiO–ZnO mixed metal oxide nanocomposite: enhanced photocatalytic and antibacterial activities. J. Mater. Sci. Mater. Electron. 29, 5459–5471 (2018). https://doi.org/10.1007/s10854-017-8513-y
Mohamed Ali A., Anwar H. A., Ahmed N. A. Investigation and characterization of simple chemical method Synthesized CdO–NiO Nancomposite. IOP Conf. Series: Journal of Physics: Conf. Series (2019) 012051.
Saadoon M. A., Ali Hassoun H., Majid H. H., Ahmed N. A., Ehab M. A., Green syntheses of CdO NPs and evaluation of their antimicrobial activities. J. Phys. Conf. Ser. 1963 (2021) 012134. https://doi.org/10.1088/1742-6596/1963/1/012134
Acknowledgements
The authors are grateful to the International Research Centre (IRC), Kalasalingam Academy of Research and Education for XRD, SEM, FTIR and UV facility provision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sasikumar, P., Revathy, M.S. & Nithiyanantham, S. Cd(OH)2 and CdO: structural, optical, electron density distribution analysis with antibacterial assay. Eur. Phys. J. Plus 137, 294 (2022). https://doi.org/10.1140/epjp/s13360-022-02492-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-022-02492-2