Abstract
Er3+-doped As2S3 glasses were synthesized using As, S and Er2S3 as raw materials. In order to produce glasses with high mechanical properties along with high solubility of rare earth ions in these glasses, different media such as water, hydraulics and heat-resistant oil were selected for quenching specimens. Heat-resistant oil was selected as optimized medium. The effects of Er content (0.5–2 wt%) on transmission, mechanical and structural properties of the glasses were investigated. By increasing Er content in the base glass, some clusters were introduced to glass field which causes reduction in transmission in infrared (from 70 to 25%) and visible ranges (from 70 to 60%). Density and microhardness of samples were increased from 2.78 to 3.26 g/cm3 and 81.5 to 91.75 Mpa, respectively, by increasing Er content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chalcogenide glasses doped with rare earth ions are promising materials for fiber amplifiers and laser applications in the IR region [1,2,3].
Low phonon energy, transparency over a wide range of wavelengths in the infrared region, readily patterned by photodarkening, high refractive index, easily prepared in bulk and thin film form, are attractive properties of chalcogenide glasses which make them suitable materials for such applications [4,5,6,7].
The low phonon energy results in two distinct characteristics; first, it affects in a reduced electron–phonon interaction and second, it generates a transparency window in IR region. Since high refractive index of these glasses (n > 2) results in a strong local electric field around the ions which induces high emission and absorption cross sections [8].
A variety of chalcogenide systems were investigated as host materials for rare earth ions like As–Ge–Se [9], Ga–Ge–Se [10], Ge–Ga–S [11], etc. Among these systems, As2S3 glassy system is considered due to its highly thermal stability, transparency up to 7 μm, easily patterned into planar waveguides by photodarkening, low absorption coefficient of ~ 0.01 cm−1 at 1535 nm [12] and readily drawn into fibers [8].
Chalcogenide glasses have been doped with different rare earth ions such as Nd3+, Ho3+, Er3+, Tm3+, Dy3+, etc. Er-doped glasses are used in the telecommunications, because Er3+-intra 4f transitions 4I13/2 → 4I15/2 (1535 nm) overlaps with low loss windows of silica fibers [13].
Since 1994 [11] till now, a variety of studies have been carried out in Er-doped As2S3 glasses quenched in water. Some of the researchers reported 4I13/2 → 4I15/2 emission (1535 nm) that was observed in the As2S3 glasses [14]. Though others reported that Er ions are not introduced into rigid networks of chalcogenide glasses, so it seems 1535 nm emission band didn’t observe in As2S3 system [15].
In this communication, we investigated Er-doped As2S3 glasses to find out the effect of Er on optical and mechanical properties of As2S3 glasses quenched in water, heat-resistant oil and hydraulic oil.
2 Experimental
The Er-doped (0.2–2 wt%) As2S3 glasses were synthesized by melting appropriate amounts of highly pour As (Sigma Aldrich, 99.999% purity), S (Sigma Aldrich, 99.99% purity) and Er2S3 (Pub Chem, 99.999% purity). The raw materials were weighted in glove box and poured into pre-cleaned silica ampoules (soaked in HF acid, distilled water and acetone, respectively, and then introduced in furnace with temperature of 700 °C for 2 h). Then ampoules sealed under vacuum (10−3 Torr). The ampoules were placed in the rocking furnace, melted at 650 °C and they kept for 8 h and subsequently quenched in different media including water with temperature 25 °C, hydraulics (Density 750 kg/m3, Flash point 220 °C, Viscosity in 100 °C is 4.6 CSt; Shell oil company) with temperature 130 °C and heat-resistant oil (Flash point 208 °C, Density 857 kg/m3, Viscosity in 100 °C is 5.1 mm2/s) with temperature 130 °C. The obtained glasses were annealed at 160 °C for 2 h to remove thermal stress. For further analysis, glass rods were cut into disks with 3 mm thickness and then polished. The glasses composition, quenching media and samples code are listed in Table 1.
The X-ray diffraction (XRD) patterns of the powdered samples were obtained by Philips X-pert-MDD system to check amorphous nature of produced specimens. X-ray pattern was obtained by Cu source emitting 0.15406 nm radiation (30 kV, step size: 0.02°, time per step: 1.2S). The FT-IR and UV–Vis spectra of the glasses were measured by Shimadzu 8400 s and UV–Vis–NIR Shimadzu 3100, respectively, for investigation of maximum transmission of the glasses in IR and Vis ranges. Densities of the obtained glasses were measured by Archimedes methods. The glass transition temperature (Tg) was measured by DSC60-Shimadzu differential scanning calorimeter (DSC). The glass powder was poured into aluminum crucible and heated up to 350 °C (10 °C/min).
The microhardness of the glasses was measured by Zwick-German Roell. The samples were under 30 g force for 15S. The measurements for each sample were repeated for 10 times. The microstructure and composition of the glasses were checked by MIRA3TESCAN-XMU field emission scanning electron microscope.
3 Results and discussion
Rare earth-doped chalcogenide glasses are usually quenched in water after melting. In comparison with other quenching media, when the melt of glasses quenched in water, structure and network of glasses are non-compress and open which causes to increase solubility of rare earth ions. Due to the high cooling rate of water, quenching in water can result in poor mechanical properties in glassy samples. The solubility of RE ions in As2S3 glasses is low [12, 16]. According to previous reports, 0.2 wt% Er was added to As2S3 base glass. Obtained composition was quenched in water after melting, subsequently annealed at 160 °C for 2 h and then glass rods were cut. The glass rods were crushed while cutting. To resolve this problem, they were quenched in hydraulics and heat-resistant oil. Since the cooling rate of these media is lower than water the glasses have high strength, therefore they didn’t crush through cutting.
Figure 1 shows the XRD patterns 0.2 wt% Er-doped As2S3 samples quenched in water, hydraulics and heat-resistant oil. The absence of any sharp peak which associated with crystalline phases in XRD patterns confirms the amorphous nature of samples. Two broad bands in the XRD pattern are related to nucleants that may grow slightly through heat treatment at crystallization temperature.
The FT-IR spectra are shown in Fig. 2. Maximum transmission of the sample that quenched in heat-resistant oil is lower than the sample quenched in hydraulic oil. In comparison with hydraulics oil, high cooling rate of the heat-resistant oil can lead to have glasses with non-compress and open structure, lower transmission and strength glasses. But on the contrary, they are less tendentious to crystallization and have more solubility of RE ions. In addition, if the melting temperature be high, there is the risk of flaming, so for security, we select heat-resistant oil as quenching medium.
XRD patterns of samples containing different amounts of Er are presented in Fig. 3. Similar to Fig. 1 all the samples have amorphous nature.
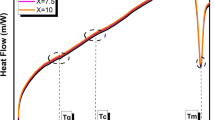
Figure 4 shows the DSC curves of As2S3 glasses doped with different amounts of Er. The glass transition temperature (Tg) of the glasses to Er concentration is shown in Fig. 5. The glass transition temperature of samples increased from 182 to 187 °C by increasing Er content from 0.5 up to 2 wt%. The glass transition temperature typically depends on mean coordination number and bonding strength. High mean coordination number and bonding strength results in high glass transition temperatures. The radius of Er ion (~ 226 pm) is significantly higher than As (~ 114 pm) and S (~ 88 pm). Therefore, it is more likely to act as a modifier in this system and reduces the mean coordination number. The melting point and bonding strength of Er2S3 (~ 1730 °C) are significantly higher than As2S3 (~ 300 °C). As it was shown, the Tg of samples was increased with increasing Er amount. So it seems that term of bonding strength is more effective than coordination number and leads to increase in Tg temperature of glasses.
Density of glasses typically depends on mean coordination number and molar mass of glass. Due to the modifier role of Er in the glass system, it was expected to be accompanied by a decrease in density. However, the density increases, because molar mass of Er2S3 is dramatically higher than As2S3. So as Er2S3 increased, the density and microhardness of the glasses were increased (Fig. 6).
The infrared transmission of the glasses containing various amount of Er is shown in Fig. 7. Maximum transmission of the glasses was reduced with increasing Er from 0.2 to 2 wt% Er. Er as a modifier can lead to produce non-compress structure and reduce maximum transmission of the samples.
The decrease in transmission is more significant in glasses with high amount of Er. As mentioned the solubility of Er ions in As2S3 glasses is low so with increasing Er2S3 some clusters can be formed in the glass matrix which caused scattering of light. This scattering results in further reduction in transmission in glasses with high amount of Er. The absorption bands in 2.92, 4.01, 6.31, 8.6 and 10.2 μm are related to O–H, S–H, H2O, CS2 and As–O, respectively.
Figure 8 shows the UV–Visible spectra of As2S3 glasses with various amounts of Er. A blueshift and a decrease in the maximum transmission are observed in transmission spectra of samples as concentration of Er is increasing. Also there are no peaks associated with Er 4f–4f transitions which mean that major part of Er creates clusters and only a little part of it can be introduced to glass structure and this minor part can’t make peaks on the transmission spectra. According to Beer–Lambert law Er as a modifier must increase maximum transmission in visible range but as it shown in Fig. 8 with increasing Er content, the maximum transmission in visible range has reduced. Due to the formation of clusters, the maximum transmission in visible range is accompanied by reduction. According to Figs. 7 and 8 it can be concluded that because of low solubility of Er ions in As2S3 glass, some clusters were formed in the glass which decrease maximum transmission in IR and visible range.
FE-SEM of As2S3 glass containing 0.5 wt% Er is shown in our previous work [16]. White clusters were formed in the matrix of glass. Based on literatures the cluster composition may be related to Er2S3 [12]. To prove the composition of clusters EDX analysis was performed. The results of EDX spectra that show the clusters and matrix compositions are mentioned in Table 2.
The results confirm the literatures and as it has been shown the matrix composition is almost As2S3 and the cluster composition is Er2S3 which means that major part of Er ions form clusters in the matrix of As2S3 glass.
4 Conclusion
The investigation of mechanical and optical properties of glasses revealed that heat-resistant oil is better quenching medium in comparison with water and hydraulics oil. Water medium causes crushing of glasses, and hydraulics oil causes to have glasses with compress structure which results in low solubility of RE ions in the glasses. Quenching in heat-resistant oil produces non-crushed and non-compress glasses with high potential of solving RE ions. By increasing Er content from 0.2 to 2 wt%, density and microhardness of the glasses were increased from 2.78 to 3.26 g/cm3 and 81.5 to 91.75 Mpa, respectively. Transmission of the glasses in IR and visible ranges is decreased because of scattering arising from clusters which were produced in the matrix of glass.
References
H. Harada, K. Tanaka, Photoluminescence from Pr3+-doped chalcogenide glasses excited by bandgap light. J. Non-Cryst. Solids 246, 189–196 (1999)
S. Kasap, K. Koughia, G. Soundararajan, M.G. Brick, Optical and photoluminescence properties of erbium-doped chalcogenide glasses (GeGaS;Er). J. Sel. Top. Quantum Electron. 14, 1353–1359 (2008)
S. Ramachandran, S.G. Bishop, Photoinduced integrated-optic devices in rapid thermally annealed chalcogenide glasses. J. Sel. Top. Quantum Electron. 11, 260–270 (2005)
V. Moizan, V. Nazabal, J. Troles, P. Houizot, J. Adam, J. Doualan, R. Moncorge, F. Smektala, G. Gadret, S. Pitois, G. Canat, Er3 + -doped GeGaSbS glasses for mid-IR fibre laser application. J. Opt. Mater. 11, 260–270 (2005)
V. Lyubin, M. Klebanov, B. Sfez, B. Ashkinadze, Photoluminescence and photodarkening effect in erbium-doped chalcogenide glassy films. J. Mater. Lett. 58, 1706–1708 (2004)
D. Turnbull, B. Aitken, S. Bishop, Broad-band excitation mechanism for photoluminescence in Er-doped Ge25Ga1.7As8.3S65 glasses. J. Non-Cryst. Solids 244, 260–266 (1999)
S. Ahmadpour, M. Rezvani, P. Bavafa, The effect of Sn on the physical and optical properties of (Se0.6As0.1Ge0.3)100−xSnx glasses. J. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 205, 258–263 (2018)
A. Galstyan, S.H. Messaddeq, V. Fortin, I. Skripachev, R. Vallee, T. Galstian, Tm3+ doped Ga–As–S chalcogenide glasses and fibers. J. Opt. Mater. 47, 518–523 (2015)
S. Bishop, D. Turnbull, B. Aitken, Excitation of rare earth emission in chalcogenide glasses by broadband Urbach edge absorption. J. Non-Cryst. Solids 266–269, 876–883 (2000)
M. Munzar, C. Koughia, D. Tonchev, K. Maeda, T. Ikari, C. Haugen, R. Decorby, J.N. McMullin, S.O. Kasap, Optical properties of Er-doped Gax(Ge0.3Se0.7)100−x glasses. J. Opt. Mater. 28, 225–230 (2006)
K. Koughia, D. Saitou, T. Aoki, M. Munzar, S. Kasap, Photoluminescence lifetime spectrum in erbium doped Ge–Ga–S glasses. J. Non-Cryst. Solids 352, 2420–2424 (2006)
S.Q. Gu, S. Ramachandran, E.E. Reuter, D.A. Tumbull, J.T. Verdeyen, S.G. Bishop, Photoluminescence and excitation spectroscopy of Er-doped As2S3 glass: novel broad band excitation mechanism 77, 3365–3371 (1994)
A.J. Kenyon, Recent developments in rare-earth doped materials for optoelectronics. Prog. Quantum Electron. 26, 225–284 (2002)
R.E. Brown, As2S3:Er3+: spectroscopy and spectral hole burning. B.S. thesis, Georgia State University (1999)
Q. Jiao, G. Li, L. Li, C. Lin, G. Wang, Z. Liu, S. Dai, T. Xu, Q. Zhang, Effect of gallium environment on infrared emission in Er3+-doped gallium–antimony–sulfur glasses. Sci. Rep. 7, 1–8 (2017)
A. Yousefi Dizaj, M. Rezvani, The investigation of optical and mechanical properties of Er3+ doped Ga-As-S glasses quenched in different medias. J. Infrared physics and technology 93, 199–204 (2018)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezvani, M., Yousefi Dizaj, A., Rahimian, A. et al. The effect of Er on optical and mechanical properties of As2S3 glasses quenched in different media. Eur. Phys. J. Plus 135, 532 (2020). https://doi.org/10.1140/epjp/s13360-020-00502-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-020-00502-9