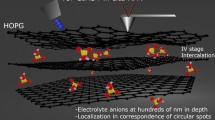
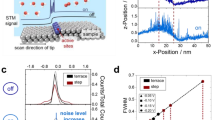
Abstract
In situ atomic force microscopy (AFM) measures, with the scanner head immersed inside a liquid (namely an electrolyte), are—generally speaking—not easy to be acquired in real time, i.e., during electrochemical processes, the immersion of a massive and wide system (with respect to the electrochemical cell mean radius) can significantly perturb the electrolyte and the I/V profile of the electrochemical characterization (CV). Despite this fact, the information that can be obtained from an electrode morphological inspection is very precious, and the coupling between an AFM and an electrochemical cell is in any case successful. In this work, we discuss the surface morphological evolution of a graphite electrode when immersed inside an aqueous NaOH electrolyte. 1.0 M sodium hydroxide added inside electrochemical baths was shown to promote the intercalation of ions between the stratified graphite structure or graphene layers. Possible consequences of diluted NaOH electrolyte on the quality of the graphite basal plane have been never considered in detail yet. By means of an in situ AFM, we show the effects of CVs on the surface morphology of the graphite basal plane. Our result shows that a 1.0 M NaOH solution, generally used for promoting intercalation, changes the electrode surface quality as a consequence of a partial carbon corrosion.





Similar content being viewed by others
References
J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng, Y. Li, H. Liu, S. Dou, Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries. Adv. Sci. 4, 1700146 (2017)
L.M. Viculis, J.J. Mack, O.M. Mayer, H. Thomas Hahn, R.B. Kaner, Intercalation and exfoliation routes to graphite nanoplateles. J. Mater. Chem. 15, 974 (2005)
G. Bussetti, R. Yivlialin, D. Alliata, A. Li Bassi, C. Castiglioni et al., Disclosing the early stages of electrochemical anion intercalation in graphite by a combined atomic force microscopy/scanning tunneling microscopy approach. J. Phys. Chem. C 120, 6088 (2016)
W. Rüdorff, Graphite intercalation compounds. Adv. Inorg. Chem. Radiochem. 1, 223 (1959)
M. Scarselli, G. Ercolani, P. Castrucci, D. Monti, G. Bussetti, M. Russo, C. Goletti, P. Chiaradia, R. Paolesse, M. De Crescenzi, Surf. Sci. 601, 2607 (2007)
V. Nicolosi, M. Chhowalla, M.G. Kanatzidis, M. Strano, J.N. Coleman, Liquid exfoliation of layered materials. Science 21, 1226419 (2013)
Z.Y. Xia, S. Pezzini, E. Treossi, G. Giambastiani, F. Corticelli, V. Morandi, A. Zanelli, V. Bellani, V. Palermo, The exfoliation of graphene in liquids by electrochemical, chemical and sonication-assisted techniques: a nanoscale study. Adv. Funct. Mater. 23, 4684R (2013)
R. Yivlialin, G. Bussetti, L. Magagnin, F. Ciccacci, L. Duò, Temporal analysis of blister evolution during anion intercalation in graphite. Phys. Chem. Chem. Phys. 19, 13855 (2017)
R. Yivlialin, L. Magagnin, L. Duò, G. Bussetti, Blister evolution time invariance at very low electrolyte pH: H\(_2\)SO\(_4\)/graphite system investigated by electrochemical atomic force microscopy. Electrochim. Acta 276, 352 (2018)
M. Jagadeesh, G. Bussetti, A. Calloni, R. Yivlialin, L. Brambilla et al., Incipient anion intercalation of highly oriented pyrolytic graphite close to the oxygen evolution potential: a combined X-ray photoemission and Raman spectroscopy study. J. Phys. Chem. C 123, 1790 (2019)
A. Kavtun, E. Treossi, N. Mirotta, A. Scidà et al., Benchmarking of graphene-based materials: real commercial products versus ideal graphene. 2D Mater. 6, 025006 (2019)
R. Yivlialin, G. Bussetti, M. Penconi, A. Bossi, F. Ciccacci, M. Finazzi, L. Duò, Vacuum-deposited porphyrin protective films on graphite: electrochemical atomic force microscopy investigation during anion intercalation. ACS Appl. Mater. Interfaces 9, 4100 (2017)
W.W. Liu, J.N. Wang, Direct exfoliation of graphene in organic solvents with addition of NaOH. Chem. Commun. 24, 6888 (2011)
Y. Yi, G. Weinberg, M. Prenzel, M. Greiner, S. Heumann, S. Becker, R. Schlögl, Electrochemical corrosion of a glassy carbon electrode. Catal. Today 295, 32 (2017)
G. Inzelt, Pseudo-Reference Electrodes, Handbook of Reference Electrodes (Springer, Berlin, 2013), p. 331
K.K. Kasem, S. Jones, Platinum as a reference electrode in electrochemical measurements. Platin. Met. Rev. 52, 100 (2008)
J. Schneir, O. Marti, G. Rammers, D. Gläser, R. Sonnenfeld, B. Drake, P.K. Hansma, Scanning tunneling microscopy and atomic force microscopy of the liquid–solid interface. J. Vac. Sci Technol. A 6, 283 (1988)
J.Y. Josefowicz, L. Xie, G.C. Farrington, Observation of intermediate CuCl species during the anodic dissolution of Cu using atomic force microscopy. J. Phys. Chem. 97, 11995 (1993)
J. Wan, Y. Hao, Y. Shi, Y.-X. Song, H.-J. Yan, J. Zheng, R. Wen, L.-J. Wan, Ultra-thin solid electrolyte interphase evolution and wrinkling process in molybdenum disulfide-based lithium-ion batteries. Nat. Comm. 10, 3265 (2019)
S. Wang, W. Zhang, Y. Chen, Z. Dai, C. Zhao, D. Wang, C. Shen, Operando study of Fe\(_3\)O\(_4\) anodes by electrochemical atomic force microscopy. Appl. Surf. Sci. 426, 217 (2017)
Y. Gu, W.-W. Wang, J.-W. He, S. Tang, H.-Y. Xu, J.-W. Yan, Q.-H. Wu, X.-B. Lian, M.-S. Zheng, Q.-F. Dong, B.-W. Mao, Electrochemical polishing of lithium metal surface for highly demanding solid-electrolyte interface. ChemElectroChem 2, 181 (2019)
L. Zheng, H.-J. Shim, Y. Cui, Y. Gao, K.-W. Lee, S.J. Hahn, S.G. Pyo, Development of a new electrochemical atomic force microscopy tool for obtaining surface morphology through electrochemical reaction. J. Nanosci. Nanotechnol. 19, 1242 (2019)
R. Yivlialin, L. Brambilla, A. Accogli, E. Gibertini, M. Tommasini, C. Goletti, M. Leone, L. Duò, L. Magagnin, C. Castiglioni, G. Bussetti, Evidence of graphite blister evolution during the anion de-intercalation process in the cathodic regime. Appl. Surf. Sci. (in press)
X. Duan, F. Xu, Y. Wang, L. Chang, Study of electrochemical oxidation of m-nitrophenol on various electrodes using cyclic voltammetry. Iran. J. Chem. Chem. Eng. 37, 129 (2018)
D. Alliata, R. Kötz, H. Siegenthaler, In situ AFM study of interlayer spacing during anion intercalation into HOPG in aqueous electrolyte. Langmuir 15, 8483 (1999)
D. Alliata, P. Häring, O. Haas, R. Kötz, H. Siegenthaler, Anion intercalation into highly oriented pyrolytic graphite studied by electrochemical atomic force microscopy. Electrochem. Commun. 1, 5 (1999)
C.A. Goss, J.C. Brumfield, E.A. Irene, R.W. Murray, Imaging the incipient electrochemical oxidation of highly oriented pyrolytic graphite. Anal. Chem. 65, 1378 (1993)
R. Yivlialin, G. Bussetti, L. Brambilla, C. Castiglioni, M. Tommasini, L. Duò, M. Passoni, M. Ghidelli, C.S. Casari, A. Li Bassi, Microscopic analysis of the different perchlorate anions intercalation stages of graphite. J. Phys. Chem. C 121, 14246 (2017)
R. Piner, R.S. Ruoff, Cross talk between friction and height signals in atomic force microscopy. Rev. Sci. Instrum. 73, 3392 (2002)
M. Campione, S. Trabattoni, M. Moret, Nanoscale mapping of frictional anisotropy. Tribol. Lett. 45, 219 (2012)
M. Campione, G.C. Capitani, Subduction-zone earthquake complexity related to frictional anisotropy in antigorite. Nat. Geosci. 6, 847 (2013)
F. Scholz, Electroanalytical methods, guide to experiments and applications (Springer, Berlin, 2010)
Acknowledgements
This work was realized at the Solid–Liquid Interface and Nanomicroscopy (SoLINano) lab, which is an inter-Departmental laboratory financed and supported by the Politecnico di Milano. Authors also thank the Regione Lombardia—Fondazione Cariplo joint project ‘SmartMatLab Centre’ where vacuum-deposited thin films were prepared; the Project “I-ZEB Verso Edifici Intelligenti a Energia Zero per la crescita della città intelligente” in the framework of ‘Accordo Quadro tra Regione Lombardia e Consiglio Nazionale delle Ricerche’ and the Project PRIN2017 “3D-FARE: Functional 3D Architectures for Electrochemiluminescence applications—Prot.\(\hbox {n}^\circ \) 2017FJCPEX.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bussetti, G., Campione, M., Bossi, A. et al. In situ atomic force microscopy: the case study of graphite immersed in aqueous NaOH electrolyte. Eur. Phys. J. Plus 135, 329 (2020). https://doi.org/10.1140/epjp/s13360-020-00333-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-020-00333-8