Abstract
Coumarins, a subgroup of colorless and crystalline oxygenated heterocyclic compounds originally discovered in the plant Dipteryx odorata, were the subject of a recent study investigating their quantitative structure–activity relationship (QSAR) in cancer pharmacotherapy. This study utilized graph theoretical molecular descriptors, also known as topological indices, as a numerical representation method for the chemical structures embedded in molecular graphs. These descriptors, derived from molecular graphs, play a pivotal role in quantitative structure–property relationship (QSPR) analysis. In this paper, intercorrelation between the Balban index, connective eccentric index, eccentricity connectivity index, harmonic index, hyper Zagreb index, first path Zagreb index, second path Zagreb index, Randic index, sum connectivity index, graph energy and Laplacian energy is studied on the set of molecular graphs of coumarins. It is found that the pairs of degree-based indices are highly intercorrelated. The use of these molecular descriptors in structure–boiling point modeling was analyzed. Finally, the curve-linear regression between considered molecular descriptors with physicochemical properties of coumarins and coumarin-related compounds is obtained.
Graphical abstract
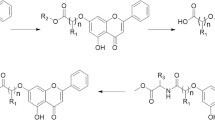
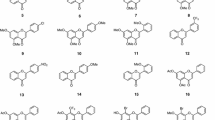
Some of the coumarin-related anti-cancer compounds considered in this study












Similar content being viewed by others
Data Availability
There are no data associated with this manuscript.
References
M. Arockiaraj, F.J.H. Campena, A.B. Greeni, M.U. Ghani, S. Gajavalli, F. Tchier, A.Z. Jan, QSPR analysis of distance-based structural indices for drug compounds in tuberculosis treatment. Heliyon 10, e23981 (2024)
M. Arockiaraj, A.B. Greeni, A.A. Kalaam, Comparative analysis of reverse degree and entropy topological indices for drug molecules in blood cancer treatment through QSPR regression models. Polycycl. Aromat. Compd. (2023). https://doi.org/10.1080/10406638.2023.2271648
J.S. Junias, J. Clement, M.P. Rahul, M. Arockiaraj, Two-dimensional phthalocyanine frameworks: topological descriptors, predictive models for physical properties and comparative analysis of entropies with different computational methods. Comput. Mater. Sci. 235, 112844 (2024)
D. Paul, M. Arockiaraj, K. Jacob, J. Clement, Multiplicative versus scalar multiplicative degree based descriptors in QSAR/QSPR studies and their comparative analysis in entropy measures. Eur. Phys. J. Plus 138, 323 (2023)
J. Bruneton, Immunotoxicity of Epicutaneously Applied Anti-Coagulant Rodenticide Warfarin (Intercept Ltd., Hampshire, 1999), pp.245–263
J. Bruneton, Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd edn. (Intercept Ltd., Hampshire, 1999), pp.263–277
A. Lacy, R. O’Kennedy, Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 10, 3797–3811 (2004)
T. Ojala, Biological Screening of Plant Coumarins. Ph.D. Thesis (University of Helsinki, Helsinki, Finland, 2001)
B. Lake, Synthesis & pharmacological investigation of 4-hydroxy coumarin derivatives & shown as anti-coagulant. Food Chem. Tox. 3, 412–423 (1999)
R.D.H. Murray, J. Mendez, S.A. Brown, The Natural Coumarins Occurrence, Chemistry and Biochemistry, Chichester (John Wiley and Sons Ltd., New York, 1982)
D. Cooke, B. Fitzpatrick, R. O’ Kennedy, T. McCormack, D. Egan, Coumarin: Biochemical Profile and Recent Developments, vol. 3 (John Wiley & Sons, New York, 1997), pp.311–322
D. Cooke, R. O’Kennedy, Comparison of the tetrazolium salt assay for succinate dehydrogenase with the cytosensor microphysiometer in the assessment of compound toxicities. Anal. Biochem. 274, 188–194 (1999)
K.C. Fylaktakidou, D.J. Hadipavlou-Litina, K.E. Litinas, D.N. Nicolaides, Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr. Pharm. Des. 10, 3813–3833 (2004)
D.R. Vianna, L. Hamerski, F. Figueiro, A. Bernardi, L.C. Visentin, E.N. Pires, H.F. Teixeira, C.G. Salbego, V.L. Eifler-Lima, A.M. Battastini et al., Selective cytotoxicity and apoptosis induction in glioma cell lines by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon species. Eur. J. Med. Chem. 57, 268–274 (2012)
R.G. Harvey, C. Cortex, T.P. Ananthanarayan, S. Schmolka, A new coumarin synthesis and its utilization for the synthesis of polycyclic coumarin compounds with anticarcinogenic properties. J. Org. Chem. 53, 3936–3943 (1988)
I. Kostova, G. Momekov, T. Tzanova, M. Karaivanova, Synthesis, characterization, and cytotoxic activity of new lanthanum (III) complexes of bis-coumarins. Bioinorg. Chem. Appl. 25651, 1–9 (2006)
M.A. Al-Haiza, M.S. Mostafa, M.Y. El-Kady, Synthesis and biological evaluation of some new coumarin derivatives. Molecules 8, 275–286 (2003)
B. Musiciki, A.M. Periers, P. Laurin, D. Ferroud, Y. Benedetti, S. Lachaud, F. Chatreaux, J.L. Haesslein, A. Lltis, C. Pierre et al., Improved antibacterial activities of coumarin antibiotics bearing 5\(^\prime \),5\(^\prime \)-dialkylnoviose: biological activity of RU79115. Bioorg. Med. Chem. Lett. 10, 1695–1699 (2000)
E. Kupeli, A. Tosun, E. Yesilada, Anti-inflammatory and Antinociceptive Activities of Seseli L. species (Apiaceae) growing in Turkey. J. Ethnopharmacol. 104, 310–314 (2006)
A. Tosun, E. Kupeli, E. Yesilada, Anti-inflammatory and antinociceptive activity of coumarins from Seseli gummiferum subsp. corymbosum (Apiaceae). Z. Nat. C 64c, 56–62 (2009)
J.R. Hoult, M. Paya, Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen. Pharmacol. 27, 713–722 (1996)
J. Jung, J. Kin, O.S. Park, A convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin, and 4-hydroxyquinolin-2(1H)-one. Synth. Commun. 31, 1195–1200 (2001)
G.R. Madhavan, V. Balraju, B. Malleshasm, R. Chakrabarti, V.B. Lohray, Novel coumarin derivatives of heterocyclic compounds as lipid-lowering agents. Bioorg. Med. Chem. Lett. 13, 2547–2551 (2003)
R.S. Moffet, Central nervous system depressants .VII. pyridyl coumarins. J. Med. Chem. 7, 446–449 (1964)
M. Paya, B. Halliwell, J.R. Hoult, Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 44, 205–214 (1992)
R.D. Murray, The naturally occurring coumarins. Fortschr. Chem. Org. Naturst 83, 1 (2002)
Y. Li, A. Aslam, S. Saeed, G. Zhang, S. Kanwal, Targeting highly resisted anticancer drugs through topological descriptors using VIKOR multi-criteria decision analysis. Eur. Phys. J. Plus. (2022). https://doi.org/10.1140/epjp/s13360-022-03469-x
S.M. Cohen, L.B. Ellwein, Genetic errors, cell proliferation, and carcinogenesis. Cancer Res. 51, 6493–6505 (1991)
J.H. Fentem, J.R. Fry, Species differences in the metabolism and hepatotoxicity of coumarin. Comp. Biochem. Physiol. C 104, 1–8 (1993)
B. Basavanagoud, S. Policepatil, Topological indices of some anticancer drugs. Ratio. Math. 42, 29–59 (2022)
S. Kanwal, Y. Farooq, M.K. Siddiqui, N. Idrees, A. Razzaque, F.B. Petros, Study the behavior of drug structures via chemical invariants using TOPSIS and SAW. Comput. Math. Methods Med. (2023). https://doi.org/10.1155/2023/4262299
S. Mondal, A. Dey, N. De, A. Pal, QSPR analysis of some novel neighbourhood degree-based topological descriptors. Complex Intell. Syst. 7(2), 977–996 (2021)
H.S. Ramane, K.P. Narayankar, S.S. Shirkol, A.B. Ganagi, Terminal Wiener index of line graphs. MATCH Commun. Math. Comput. Chem. 69, 775–782 (2013)
S. Shirkol, H.S. Ramane, S.V. Patil, On the acharya index of a graph. Ann. Pure Appl. Math. 11(1), 73–77 (2016)
H. Wiener, Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1(69), 17–20 (1947)
K. Xu, M. Liu, K.C. Das, I. Gutman, B. Fortula, A survey on graphs extremal with respect to distance based topological indices. Match Commun. Math. Comput. Chem. 71, 461–508 (2014)
G. Indulal, I. Gutman, A. Vijaykumar, On the distance energy of graphs. MATCH Commun. Math. Comput. Chem. 60, 461–472 (2008)
B. Zhou, N. Trinajstic, Further results on the largest eigenvalues of the distance matrix and some distance based matrices of connected (molecular) graphs. Internet Electron. J. Mol. Des. 6, 375–384 (2007)
A.T. Balaban, Highly discriminating distance-based topological index. Chem. Phys. Lett. 89, 399–404 (1982)
E. Estrada, E. Uriarte, Recent advances on the role of topological indices in drug discovery research. Curr. Med. Chem. 8(13), 1573–1588 (2001)
R.R. Huang, S. Aftab, S. Noureen, A. Aslam, Analysis of porphyrin, PETIM and zinc porphyrin dendrimers by atom-bond sum-connectivity index for drug delivery. Int. J. Interface Between Chem. Phys. (2023). https://doi.org/10.1080/00268976.2023.2214073
W. Gao, W.F. Wang, M.R. Farahani, Topological indices study of molecular structure in anticancer drugs. J. Chem. (2016). https://doi.org/10.1155/2016/3216327
M.C. Shanmukha, N.S. Basavarajappa, K.C. Shilpa, A. Usha, Degreebased topological indices on anticancer drugs with QSPR analysis. Heliyon 6, 1–9 (2020)
A.T. Balaban, Topological indices based on topological distances in molecular graphs. Pure Appl. Chem. 55, 199–206 (1983)
Yu. Guihai, Lihua Feng, On connective eccentricity index of graphs. MATCH Commun. Math. Comput. Chem. 69(3), 611–628 (2013)
V. Sharma, R. Goswami, A.K. Madan, Eccentric connectivity index: a novel highly discriminating topological descriptor for structure property and structure activity studies. J. Chem. Inf. Comput. Sci. 37, 273–282 (1997)
L. Zhong, The harmonic index on graphs. Appl. Math. Lett. 25, 561–566 (2012)
G.H. Shirdel, H. Rezapour, A.M. Sayadi, The hyper Zagreb index of graph operations. Iran. J. Math. Chem. 4, 213–220 (2013)
D. Vukicevic, S. Nikolic, N. Trinajstic, On the path-Zagreb matrix. J. Math. Chem. 45, 538–543 (2009)
M. Randic, On characterization of molecular branching. J. Am. Chem. Soc. 97, 6609–6615 (1975)
Kinkar Ch. Das, Sumana Das, Bo. Zhou, Sum-connectivity index of a graph. Front. Math. China 11, 47–54 (2016)
I. Gutman, The energy of a graph: old and new results, in Algebraic Combinatorics and Applications. ed. by A. Betten, A. Kohnert, R. Laue, A. Wassermann (Springer-Verlag, Berlin, 2001), pp.196–211
I. Gutman, B. Zhou, Laplacian energy of a graph. Linear Algebra Appl. 414, 29–37 (2006)
Funding
Sakander Hayat is supported by UBD Faculty Research Grant (No. UBD/RSCH/1.4/FICBF(b)/2022/053). Sujata T. Timmanaikar’s research is catalyzed and supported by Vision Group on Science and Technology (VGST), Karnataka Science and Technology Promotion Society (KSTePS), Department of Science and Technology, Government of Karnataka, with scheme RGS/FSanctioned Year:2021-22. National Outstanding Youth Science Fund Project of National Natural Science Foundation of China (622260-101).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the paper.
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Timmanaikar, S.T., Hayat, S., Hosamani, S.M. et al. Structure–property modeling of coumarins and coumarin-related compounds in pharmacotherapy of cancer by employing graphical topological indices. Eur. Phys. J. E 47, 31 (2024). https://doi.org/10.1140/epje/s10189-024-00427-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-024-00427-6